Physical Science Week 2
advertisement

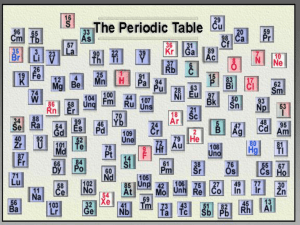
Physical Science Week 2 Periodic Table of the Elements Monday Warm Up (IAN 102) • As a group at your table: • Take the deck of cards and arrange them in a logical order. • Each person write an explanation for your system of order. Cornell Notes: Periodic Table • Periodic: Something that occurs or repeats at regular intervals Periodic Law • The law that states that the repeating chemical and physical properties of elements change periodically with the atomic numbers of the elements. Periodic Table • Rows are called periods. • Columns are called groups or families and share chemical and physical properties. • Each square has element name, chemical symbol, atomic number and atomic mass. • Elements are arranged from lowest to highest atomic number. Families or Groups • Alkali Metals (Group 1) • Alkaline – Earth Metals (Group 2) • Transition Metals (Group 3-12) – Lanthanides: shiny, reactive transition metals – Actinides: radioactive, unstable, 95+ not natural • • • • • • • Boron Group (13) Carbon Group (14) Nitrogen Group (15) Oxygen Group (16) Halogens (17) Noble Gases (18) Hydrogen – not in group 1 Tuesday Warm Up (IAN 102) • Find Gold on the periodic table. What is its atomic number, atomic mass, chemical symbol, family, and period? Video: Simply Science- Periodic Table Measuring Up P. 101-110 Versa Tiles: Periodic Table Wednesday Warm Up (IAN 102) • Compare and contrast metals, metalloids, and nonmetals. • Use a triple Venn diagram. Graphic Organizer • Create a graphic organizer that shows all the families on the periodic table. Include: – – – – – Name Group contains Reactivity Other shared properties List of elements Thursday Warm Up (IAN 102) • Compare and contrast noble gases and alkali metals. Alien Periodic Table • Use the clues given to complete the Alien Periodic Table. Friday Warm Up (IAN 102) • Describe the elements in the halogen group. • What are the typical characteristics of halogens? Review • Write 2 questions about the periodic table. • Quiz your table with your questions. • Matching: families & characteristics Periodic Table Quiz Text p. 338-339 • Background color: metal, nonmetal, metalloid • Chemical symbol color: state of matter at room temperature. Metals • Left of zigzag line • Few valence electrons • Most are solid at room temperature (except Mercury) • Shiny • Ductile- can stretch into wire • Good conductors – electric & heat transfer • Malleable – flatten without shattering Non Metals • • • • • • Right of the zigzag line Almost full valence electron shell Majority gas at room temperature Not malleable or ductile Not shiny Poor conductors Metalloids • • • • Border the zigzag line Semiconductors Half filled valence electron shells Some properties of metals and some properties of non-metals • Boron, silicon, germanium, arsenic, antimony, tellurium Chemical symbol • One capital letter with or without a second or third small letter