Metals - General Chemistry
advertisement
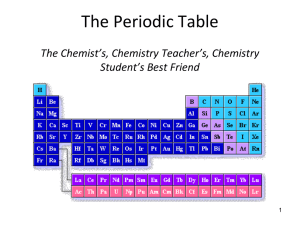
The Atom Introduction to the Periodic Table The Periodic Table • The Periodic Table of Elements is the chart that lists every known element according to their physical and chemical properties • Elements are arranged in rows called periods by increasing atomic number • Elements in the same column are said to be members of the same family or group • Elements can also be categorized in many other ways, according to placement and properties The Periodic Table Period 4 Group 13 Metallic Character Elements can also be categorized by metal, non-metal, or semimetal (also called metalloids) • • • • • • Metals Good conductors of heat/electricity Malleable Ductile Strong Lustrous Tend to lose electrons in reactions Metalloids • Intermediate properties of metals and nonmetals • Boron, silicon, germanium, arsenic, antimony, tellurium, polonium, and astatine • • • • • Non-Metals Poor conductors of heat/electricity Not malleable Not ductile Often have dull appearance Tend to gain electrons in reactions with metals Metallic Character Non-Metals (blue) Metals (grey) Metalloids (orange) Alkali Metals Alkali Metals belong to Group 1 and consist of lithium, sodium, potassium, rubidium, cesium, and francium Alkaline Earth Metals Alkaline Earth Metals belong to Group 2 and consist of beryllium, magnesium, calcium, strontium, barium, and radium. Pnictogens The Pnictogens are Group 15 (or 5A) and consist of nitrogen, phosphorus, arsenic, antimony, bismuth, and element 115 (ununpentium) Chalcogens The Chalcogens are Group 16 (or 6A) and consist of oxygen, sulfur, selenium, tellurium, polonium, and element 116 (recently named Livermorium!) Halogens The Halogens belong to Group 17 (or 7A) and consist of fluorine, chlorine, bromine, iodine, astatine, and element 117 (ununseptium) Noble Gases The Noble Gases belong to Group 18 (or 8A) and consist of helium, neon, argon, krypton, xenon, radon, and element 118 (ununoctium) Transition Metals The Transition Metals belong to Group 3-12 and also include the Rare Earth Metals (lanthanides and actinides). Lanthanides Actinides Chemistry Coffee Break Fast Facts of the Periodic Table • • • The letter “J” is the only letter that does not appear on the periodic table There is strong controversy about where to place hydrogen All elements from Polonium and on are radioactive, and Technetium (atomic number 43)! – Recently, Bistmuth (atomic number 83) was found to be slightly radioactive, but with a half-life of more than 1 billion times the age of the universe! Thus, it is generally considered stable • • • The only elements liquid under standard conditions are mercury and bromine Tungsten is the only elemental name to come from a German term (“wolfram,” hence the atomic symbol W) Scientists are working on creating element 120, which will change the shape of the periodic table • Flerovium (114) and Livermorium (112) are the latest elements to be named (May 2012). – Flerovium was first created in 1998. Despite being in Group 14, it seems to exibit Noble Gas properties – Livermorium was first detected in 2000 and so far, only about 35 atoms have been synthesized.