Enzyme Kinetics
advertisement

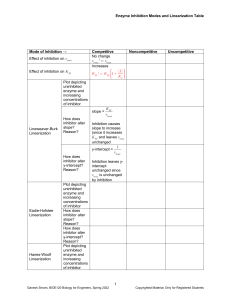
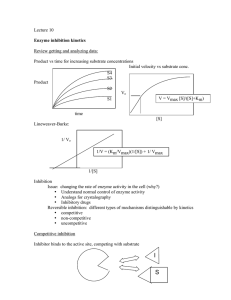
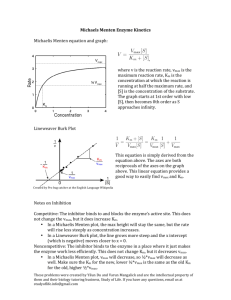
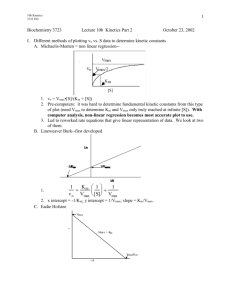
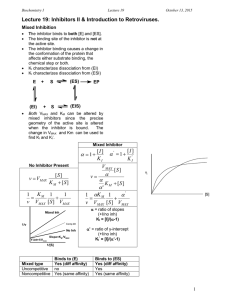
What is measurable/reported (e.g. in BRENDA)? Enzyme Kinetics i.e. characterization by assay in the test tube (typically with purified protein and through monitoring of emerging product) saturation conditions [S] >> [E] Stryer Fig. 8.12 Determining initial velocity steady-state assumption Michaelis-Menten Kinetics (slides need a few minor corrections!) k2 k1 a.k.a. kcat E + S !ES"E+P k-1 v= d[P] dt (k-2 is negligible until products start to build up) = k2[ES] Steady state conditions--[ES] remains relatively constant over the course of the rxn until S starts running out. d[ES] dt =0 k1[E][S] = k-1[ES] + k2[ES] = (k-1 + k2)[ES] Define a new constant: [E][S]/[ES] = (k-1 + k2)/ k1= KM KM[ES] = [S][E] [E] = [E]T -[ES] KM[ES] = [E]T[S]-[ES][S] [ES](KM + [S]) = [E]T[S] [E]T[S] [ES] = ------------------KM + [S] k2[E]T[S] v = -----------------KM + [S] vmax[S] v = ----------------KM + [S] vmax v = ---2 And [ES] = v/k2 Define vmax=k2[E]T Michaelis-Menten equation at [S] = KM Determining kcat and KM from “intial rate” data [S] vo 5 10 20 50 100 200 22 39 65 102 120 135 Vmax = 150-160?? Vo 150 Km = ??? 100 50 0 0 100 200 300 [S] Vo a Vmax = 150-160?? 150 100 50 0 Km = ??? 0 100 200 300 LineweaverBurk plot Vmax = 164 µM/min 1/Vo [S] y = 0.1961x + 0.0061 R2 = 0.9995 0.06 0.04 0.02 0 0 Km = 32.2 µM 0.1 0.2 1/[S] 0.3 VVP Fig. 12-4 LineweaverBurk plot competitive inhibition KM increases; Vmax unchanged + inhibitor Vmax no inhibitor 1/Vo 100 Vo 75 50 + inhibitor 25 0 0 10 KM [S] 20 no inhibitor 1/[S] 30 -1/KM competitive inhibition VVP Fig. 12-7 modifying factor # mixed and uncompetitive inhibition Vmax decreases uncompetitive inhibition (rare) VVP Fig.12-8 mixed competitive inhibition (a.k.a. non-competitive inhibition if KI ! K’I) VVP Fig. 12-9 Summary of simple inhibition models Inhibitor binds at Effect on Vmax Effect on KM other competitive Active site None Apparent KM increases uncompetitive Allosteric site after S binds decreases Apparent KM decreases noncompetitive (or “mixed”) Allosteric site (to E or ES) decreases varies Overcome inhibition at high [S] noncompetitive is special case: no KM change Catalytic Mechanism Determination 1) kinetic analysis - what is the kinetic “signature”? - mode of inhibition revealed - determine rates for individual steps - does order of addition matter? (sequential vs. Ping-Pong) 2) active site modification (irreversible inhibitors) - derivatize protein; identify the modified sidechain(s) 3) structure determination (e.g. RNase A, lysozyme, serine proteases) Some Peroxidases use heme prosthetic groups (e.g. cytochrome c peroxidase uses heme b EC 1.11.1.5, PDB 2CYP) (Fe is 2+/II or 3+/III or 4) +/IV or 6+/VI) (metal co-ordination = donated bonds!) (hemoglobin/myoglobin; cytochrome c peroxidase) _ _ His-N His-N His-N - | O-O|- _ _ - | O-OH Enzymatic redox cycles typically involve two redox reactions “intended” reaction cofactor is recycled (two ways shown) Interesting: In cytochrome c peroxidase, the radical seems to be forming on a tryptophan (could also be in the heme system) radical • (- charge symbolizes that system is one edeficient) Are these residues conserved? (e.g. in PIRSF family around PDB:2CYP) (http://chem-faculty.ucsd.edu/kraut/projects.html) Indications for this come largely from a crystal structure of a dioxygen (O2) complex Many important reaction steps are proton transfer (rather than e--transfer) Indolyl cation radical on Trp51 seems to be stabilized by the enzyme (much less labile than in solution - up to 4h half-life!) (Vit: Niacin) NAD+ / NADH NADP+ / NADPH add 2H+ + 2elose 1H+ (Vit: Riboflavin) FAD / FADH2 FMN / FMNH2 add 2H+ + 2e- Redoxcoenzymes Also: Other folate/THF-derivatives Note in closing: Controversy regarding precise mechansim(s) of redox reactions (e.g. using NAD+/NADH) • direct (bio-)hydride transfer (i.e. H- “ion”) • direct electron transfer (via free radical = 1e-) • intermediates enabling transfer via covalent bond … though as bioinformaticians we don’t care all that much :-)