NovaMin and Gingivitis
advertisement

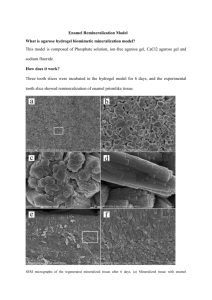
NovaMin What is NovaMin®? A “high-tech” active ingredient used in the most innovative professional dental products. Material Description NovaMin is a breakthrough remineralization technology. A tasteless white powder form. NovaMin amplifies the natural protective and repair mechanisms for teeth and gums. Extensively proven in clinical and scientific studies at leading dental schools around the world. Technical Breakdown Comprised of calcium, sodium, phosphorous and silica, all natural elements found in the body Chemical name is Calcium Sodium Phosphosilicate NovaMin is recognized as the only man made ingredient that directly leads to the formation of new tooth mineral (HCA) What Does NovaMin® Do? Repairs Surface Lesions Forms biomimetic HCA on tooth dentin and enamel surface lesions Reduces Sensitivity Occludes dentin tubules: forms a protective biomimetic HCA layer. Improves Gingival Health Antimicrobial properties & Anti-inflammatory properties reduce gingivitis. The NovaMin® Solution Hard Tissue Benefits The NovaMin® Solution Soft Tissue Benefits NovaMin: How it Starts NovaMin is also called “calcium sodium phosphosilicate”. It is made from elements naturally found in healthy teeth and bones: Calcium ( ), Phosphorus ( ), Sodium ( ) and Silicon ( ) Step 1: pH Elevation +H2O NovaMin reacts with saliva. Na+ ions release, elevating pH into the range essential for HCA formation. 8.0-8.5 pH Step 2: Mineral Release Ca2+ and P5+ ions are released, saturating saliva with the ions needed for remineralization. Ca2+ P5+ Step 3: Bacterial Kill Na+ and Ca2+ ions overwhelm bacterial cells’ ability to regulate their water content, leading to lysis (cell death) of harmful bacteria in the mouth. F. Nucleatum S. Mutans A.Naeslundii -99.99% S. Sanguis -99.999% -99.999% -99.99999% Tooth Mineral Structure - Hydroxy-carbonate Apatite (“HCA”) Step 4: Crystal Building Enamel Crystal Prism Demineralized lesions attract Ca2+ and P5+ ions, where they build new HCA crystal to remineralize the defect from the bottom up. Tooth Mineral Structure - Hydroxy-carbonate Apatite (“HCA”) Step 5: Time Release Enamel Crystal Prism NovaMin particles adhere to tooth surfaces for up to 7 days, continuing to release Na+, Ca2+, and P5+ ions to maintain elevated pH at the tooth surface and foster remineralization over time. Remineralization of Dentin & Enamel In-vitro Study – In-Situ Models After Treatment Before Treatment Surface Lesion In-vitro Surface Abrasion In-Situ Exposed Dentin In-vitro NovaMin Product Focuses • Sensitivity: Faster & better results Addresses symptom, not cause. • Gingivitis: Faster results Less controversial ingredient • Root Caries/Remineralization: Adult caries predominantly related to hyposalivation. NovaMin directly addresses this etiology. NovaMin & Sensitivity NovaMin Therapy Paste Results Clinical Results Vs. Placebo VAS Score Averaged Across Methods 8 7 6 5 4 3 2 1 0 NovaMin Control p<0.001 Baseline 1 week 2 weeks 3 weeks 4 weeks NovaMin & Sensitivity NovaMin Therapy Paste Results Clinical Results Vs. Other Actives NovaMin Vs. Potassium Nitrate 8.0 VAS Water p<0.05 6.0 Potassium Nitrate 4.0 Stannous Fluoride NovaMin 2.0 0.0 0 2 4 Weeks 12 NovaMin vs. Fluoride Remineralization of Dentin NTI-UCSF In-vitro Study – Dentin Remin/Demin Model 1400 Product Tested 1200 7.5% NM/1100 ppm MFP 1 800 7.5% NM 2 600 Company CR 5000 ppm NaF 3 1000 Delta Z Code 400 Company CG 1.1 ppm MFP 4 0% NM/O ppm F 5 200 0 1 2 3 Product Code Tested 4 5 NovaMin and Gingivitis Anti-Microbial Properties Log Reduction in Microbial CFU's - 2 Minute Exposure to 1:3 Dilution Bacterial Species 0 S mutans S sanguis F nucleatum A naeslundii -1 Log Reduction in Viability -2 -3 -4 -5 -6 -7 -8 -9 NovaMin Non Aqueous Toothpaste NovaMin Powder Colgate Red Box Toothpaste NovaMin and Gingivitis Pilot Clinical Study – Gingival Bleeding Index Summary of Gingival Index Findings 1.4 p<0.01 1.2 GBI 1 0.8 -59% 0.6 NovaMin Placebo 0.4 0.2 0 baseline 6 week Time Period NovaMin and Gingivitis Pilot Clinical Study – Plaque Index Summary of Plaque Index Findings 1.8 1.6 p<0.025 1.4 PLI 1.2 1 NovaMin Placebo 0.8 0.6 0.4 0.2 0 baseline 6 week Time Period NovaMin/Fluoride Enamel Remineralization In-vitro Study – Remin/Demin Model Surface Lesion ~ 100µm Sound Enamel Demineralized Untreated Sample Sound Enamel Fluoride Dentifrice Treated Sample Sound Enamel NovaMin Dentifrice + F Treated Sample NovaMin/Fluoride Enamel Remineralization In-vitro Study – Remin/Demin Model Lesion Area Lesion Area (sq. microns x1000) 40 p<0.001 30 -30% -45% 20 10 0 Demin F paste Treatment Group F + NovaMin