Balancing Equations & Reaction Types Chemistry Worksheet
advertisement

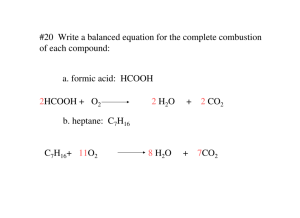
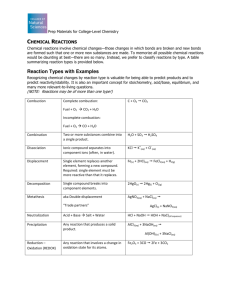
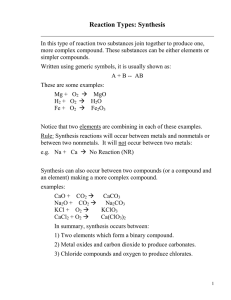
#20 Write a balanced equation for the complete combustion of each compound: a. formic acid: HCOOH 2HCOOH + O2 2 H2O + 2 CO2 b. heptane: C7H16 C7H16+ 11O2 8 H2O + 7CO2 21. Write a balanced equation for the complete combustion of glucose: C6H12O6 C6H12O6 + 6 O2 6 H2O + 6 CO2 #22 What are the five types of chemical reactions? #23 What are the keys to predicting the products of the five general types of reactions? 1. combination: Two elements combine to form one new compound. 2. decomposition: one compound breaks up into its elements. 3. single replacement: one element replaces another in a compound. 4. double replacement: two compounds exchange ions to form two new compounds. 5. combustion: a fuel reacts with oxygen gas to form carbon dioxide and water vapor. #24 Classify each reaction and balance the equations: a. 2 C3H6 + 9 O2 63 CO2 +63 H2O combustion b. 2 Al(OH)3 Al2O3 + 3 H2O decomposition c. d. 4 Li + O2 2 Li2O combination Zn + 2 AgNO3 single replacement 2Ag + Zn(NO3)2 #25 Which of the five general types of reactions would most likely occur, given each set of reactants? What are the probable products? a. an aqueous solution of two ionic compouds double replacement is most likely. The most likely products are two new compounds. b. a single compound a decomposition is most likely. The elements from which this compound is composed are the likely products. c. two elements a combination is most likely. The likely product will be a “combination” of the two elements d. oxygen and a compound of carbon and hydrogen combustion. The likely products are CO2 and H2O #26 complete and balance an equation for each reaction: a. CaI2 + Hg(NO3)2 a. CaI2 + Hg(NO3)2 HgI2(s) ? (HgI2 precipiates) + Ca(NO3)2 b. Al + Cl2 ? b. 2Al + 3Cl2 2AlCl3 c. Ag c. 2Ag d. C2H2 + HCl + 2HCl + O2 d. 2C2H2 e. MgCl2 e. MgCl2 ? 2AgCl + + ? 5O2 4CO2 ? H2(g) Mg + Cl2 + 2H2O #27 What are the three types of products that result in double-replacement reactions?