Chemical Reaction Types: Synthesis, Decomposition & More
advertisement
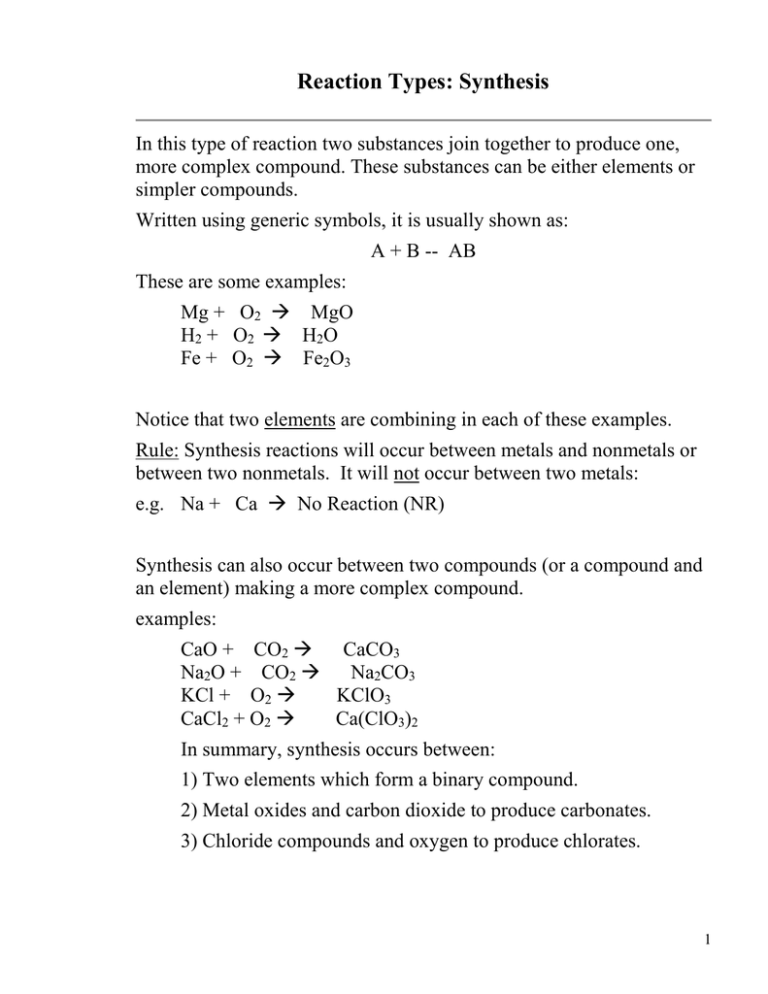
Reaction Types: Synthesis In this type of reaction two substances join together to produce one, more complex compound. These substances can be either elements or simpler compounds. Written using generic symbols, it is usually shown as: A + B -- AB These are some examples: Mg + O2 MgO H2 + O2 H2O Fe + O2 Fe2O3 Notice that two elements are combining in each of these examples. Rule: Synthesis reactions will occur between metals and nonmetals or between two nonmetals. It will not occur between two metals: e.g. Na + Ca No Reaction (NR) Synthesis can also occur between two compounds (or a compound and an element) making a more complex compound. examples: CaO + CO2 CaCO3 Na2O + CO2 Na2CO3 KCl + O2 KClO3 CaCl2 + O2 Ca(ClO3)2 In summary, synthesis occurs between: 1) Two elements which form a binary compound. 2) Metal oxides and carbon dioxide to produce carbonates. 3) Chloride compounds and oxygen to produce chlorates. 1 Reaction Types: Decomposition During decomposition, one compound is broken apart into two(or more) pieces. These pieces can be either elements or simpler compounds. Written using generic symbols, it is usually shown as: AB A + B Note: These are the reverse of synthesis reactions. HgO H2O Fe2S3 Hg + O2 H2 + O2 Fe + S Rule: Decomposition reactions will occur as long as sufficient energy is supplied. The energy will usually be in the form of heat, electricity or light. Notice that in every decomposition reaction, there is only one substance on the left-hand (reactant) side. Don't forget that!! Decomposition reactions may also split one compound into two simpler compounds (or compound and an element) as in these examples: CaCO3 CaO + CO2 Na2CO3 Na2O + CO2 KClO3 KCl + O2 Ba(ClO3)2 BaCl2 + O2 2 Here are three categories of decomposition reactions: 1) All binary compounds will break down into their elements. 2) All carbonates break down to a metal oxide and carbon dioxide. 3. Chlorates will break down to a binary chloride compound and oxygen. Complete the following examples: 1) NaCl 2) NaClO3 3) Li2S 3 4) Li2CO 3 Acids and Bases Here are two other examples of Synthesis Reactions: Metal oxides + water bases. CaO + Na2O + H2O Ca(OH)2 H2O NaOH Nonmetal oxides + water SO2 + H2O N2O5 + H 2O acids. H2SO3 HNO3 4 Reaction Types: Single Replacement During single replacement, one element replaces another element in a compound. There are two different possibilities: 1) One cation replaces another cation. Using generic symbols, it is written as: AB + X XB + A Element X has replaced A to form a new compound XB and the free element A. Remember that A and X are both cations (positively-charged ions) in this example. Rule: The element doing the replacing must be more active than the element in the compound. Some examples are: Cu + AgNO3 Ag + Cu(NO3)2 Cu + Fe(NO3)2 NR (Cu is less active than Fe) Zn + HCl ZnCl2 + H2 (demo) Al + CuCl2 AlCl3 + Cu (demo) 5 The second possibility of replacement: 2) One anion replaces another. Written using generic symbols, it is: AB + Y AY + B Element Y has replaced B to form a new compound AY and the free element B. Remember that B and Y are both anions (negatively-charged ions) in this example. NaBr + Cl2 KI + Br2 NaCl + KBr + Br2 I2 Once again, the element doing the replacing must be more active. Notice: In a single replacement reaction, one reactant is always an element. The other reactant is a compound. Solve these examples: 1) ZnS + O2 2) KBr + H2 3) Ni + 4) NaI CoCl2 + Br2 6 Reaction Types: Double Replacement During double replacement, the cations and anions of two different compounds switch places. Using generic symbols, it is written as: AB + XY -- AY + XB A and X are the cations in this example, with B and Y being the anions. Some examples are: AgNO3 + SrS + KOH + Na2SO4 HCl Ag2SO4 + SrCl2 + H2SO4 K2SO4 + NaNO3 H2S(g) H2O Rule: In order for a double replacement reaction to occur, the reaction must begin with soluble reactants that produce at least one of the following three products: an insoluble product (a precipitate) a gas water 7 An equation that does not meet the above rule will result in no reaction For example: Cu(NO3)2(aq) + KClO3(aq) Cu(ClO3)2(aq) +KNO3(aq) Although the first requirement is met because both reactants are soluble, both of the products are also soluble and neither is a gas or water. Therefore, the reaction would not occur. Cu(NO3)2(aq) + KClO3(aq) NR Solve these examples: 1) NaNO3 + HgCl2 2) Ca(OH)2 + HCl 3) Al2(CO3)3 + H2SO4 4) Pb(NO3)2 + K2S 8 Reaction Types: Combustion Combustion, in its most general sense, can mean the reaction of oxygen gas (O2) with anything. However, we will define combustion to mean the reaction of oxygen with a compound containing carbon and hydrogen (hydrocarbon). A common synonym for combustion is burn. Written using generic symbols, it is usually shown as: CxHy + O2 CO2 + H2O These are some examples: CH4 + O2 CO2 + H 2O C2H6 + O2 CO2 + H 2O C6H12O6 + O2 CO2 + H 2O Notice that some compounds contain carbon, hydrogen AND oxygen. However, the products are the same, in every reaction! Reactions vary a bit when nitrogen (burns to form NO2) or sulfur (burns to form SO2) are part of the formula. C21H24N2O4 + O2 CO2 + H2O + C2H5SH + O2 CO2 + H2O + SO2 NO2 Solve these sample combustions: 1) 2) 3) C7H6O + O2 CH3COCH3 + O2 H2C2O4 + O2 A combustion reaction with insufficient oxygen yields carbon monoxide (CO) rather than carbon dioxide (CO2). (A source of air pollution) 9






