Oxidation notes
advertisement

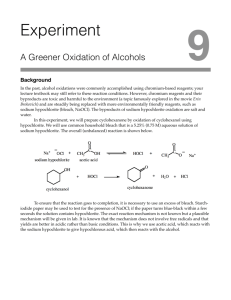
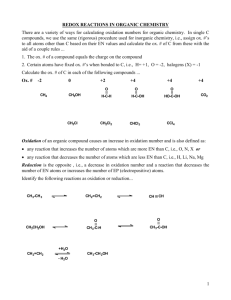
Dehydration Expt. – GC Results • Handed back chromatograms from last week’s Dehydration experiment… • Should have seen 3 peaks (one for each alkene product), BUT only see 2 peaks… – Poor resolution – “Missing” peak is actually covered up by large peak. • Don’t worry about assigning what peak is what, just get total % alkenes so that you can multiply it by your mass to get actual yield (so impurities count this lab). • Follow the rest of instructions in prelab powerpoint slides from Dehydration Expt. Oxidation of Cyclododecanol Oxidation is defined as… • Loss of electrons • Increase in oxidation number -These work well for metallics • Addition of an oxygen atom • Removal of 2 hydrogen atoms Calculating Oxidation Number • For carbon: 1. Assign oxidation numbers to the atoms attached to the carbon using the following values: 1. 2. 3. +1 for hydrogen -1 for halogens, nitrogen, oxygen, and sulfur 0 for carbon 2. Sum the oxidation numbers of these atoms. 1. If an atom is double bonded, multiply its oxidation number by 2; If triple bonded, multiply by 3. 3. Determine that the sum of the number from step 2 and the oxidation number of the carbon atom equals the charge on the carbon atom, which is zero unless it bears a +/- charge. Calculating Oxidation Number • For other atoms (esp. those in polyatomic ions) 1. Hydrogen must always have an oxidation number of +1. 2. Oxygen must always have an oxidation number of -2. 3. The sum of all the oxidation numbers must be zero, so then solve for the atom under consideration. Oxidizing Reagents Common examples from the text – 1. KMnO4 2. H2CrO4 or H2SO4 and CrO3 3. HNO3 4. Pyridinium chlorochromate 5. Hypochlorous acid – Sodium hypochlorite (we will be using this today) is the main ingredient in household bleach. PROCEDURE: 1. Cyclododecanol is dissolved in acetone to which acetic acid is added. 2. The solution is warmed to 45oC and held there using a water bath. Bath temperature monitored 40-50oC 3. Sodium hypochlorite (4.5mL) is added slowly over about 20 minutes. Note: Carbon goes from 0 to +2 (2 e- change) Chlorine goes from +1 to -1 (2 e- change) 5. To ensure an excess of hypochlorite, test using starch/iodide paper (which consists of KI and starch). Cl goes from +1 to -1 (2 e- change) I goes from -1 to 0 (1 e- change) Iodine reacts with polysaccharides such as starch to form a complex ion which is blue-black in color. 6. Add more bleach as needed to ensure an excess. 7. Transfer to a separatory funnel – wash the flask with ether and add that to the funnel – shake and separate layers. Keep both layers. (Ether extraction of aq. layer) 8. Extract the aq. layer with another 5 mL portion of ether and combine with the 1st 5 mL of ether. The product should be in the ether layer. 9. Wash the combined ether layers with: - bicarbonate – to remove (acetic) acid NaHCO3 + HC2H3O2 = NaC2H3O2 + H2O + CO2(g) - bisulfite – to remove sodium hypochlorite ClO- + NaHSO3 = Cl- + NaHSO4 - saturated aq. NaCl solution (salting out) 10. Dry over anhydrous Na2SO4 for at least 15 minutes. 11. Carefully pipette your product into another (preweighed, empty) vial to separate it from the desiccant. 12. Weigh the combined mass (and then subtract out the mass of the empty vial to get the mass of your product). Confirmatory Tests How do you know you have performed a chemical change? Ketones will react with 2,4-dinitrophenylhydrazine to give a precipitate ranging in color from yellow to red; alcohols will not. Test both the starting compound and the product by mixing a drop of each reagent and observing any precipitate. To Prepare Your NB… • 7 Chemicals: cyclododecanol, acetone, glacial acetic acid, sodium hypochlorite, diethyl ether, cyclododecanone, 2,4-dinitrophenylhydrazine • 5 Chemical Reactions: – – – – – Overall oxidation reaction How HClO is generated in solution with acetic acid Neutralization of acetic acid with sodium bicarbonate Bisulfite wash to remove hypochlorite Confirmatory test (but CUSTOMIZE to our specific reaction) • Use the procedure in this powerpoint as a guide and pgs. 543-544 to help you write a procedure in your NB . • Figure 2.34 (b) on pg. 53, except we’re using water not oil. Calculations 1. Calculate the theoretical yield – remember hypochlorite is in excess. 2. Calculate the % yield based on the mass of product that you recovered.