Experiment 9 - A Greener Oxidation of Alcohols
advertisement
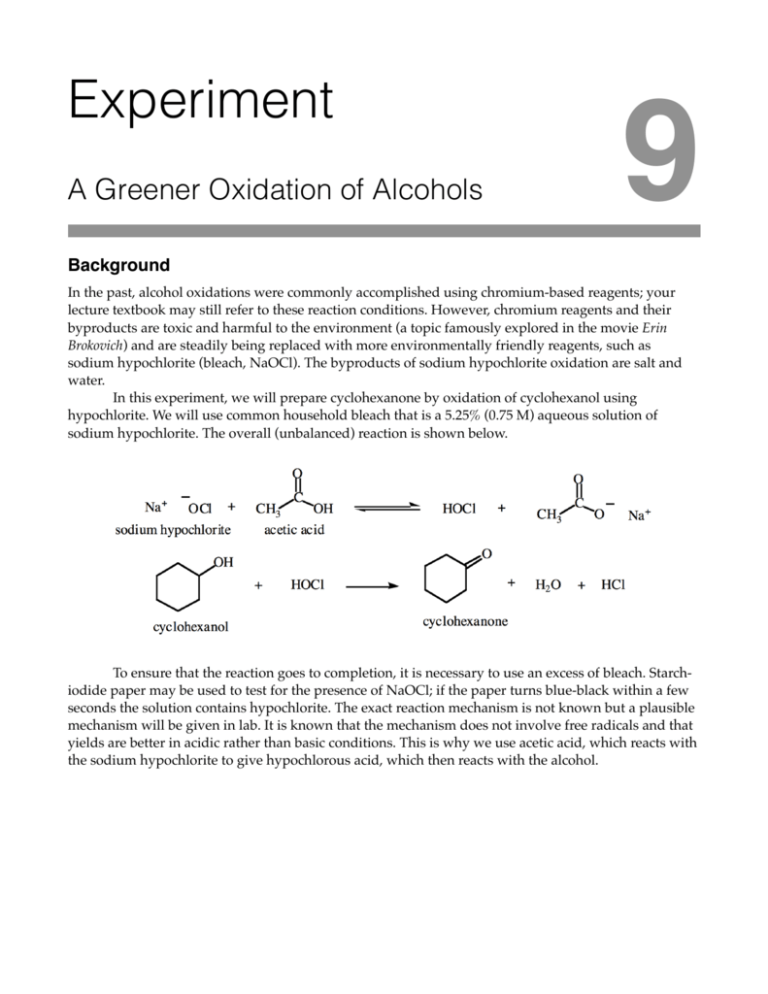
Experiment A Greener Oxidation of Alcohols 9 Background In the past, alcohol oxidations were commonly accomplished using chromium-based reagents; your lecture textbook may still refer to these reaction conditions. However, chromium reagents and their byproducts are toxic and harmful to the environment (a topic famously explored in the movie Erin Brokovich) and are steadily being replaced with more environmentally friendly reagents, such as sodium hypochlorite (bleach, NaOCl). The byproducts of sodium hypochlorite oxidation are salt and water. In this experiment, we will prepare cyclohexanone by oxidation of cyclohexanol using hypochlorite. We will use common household bleach that is a 5.25% (0.75 M) aqueous solution of sodium hypochlorite. The overall (unbalanced) reaction is shown below. ! To ensure that the reaction goes to completion, it is necessary to use an excess of bleach. Starchiodide paper may be used to test for the presence of NaOCl; if the paper turns blue-black within a few seconds the solution contains hypochlorite. The exact reaction mechanism is not known but a plausible mechanism will be given in lab. It is known that the mechanism does not involve free radicals and that yields are better in acidic rather than basic conditions. This is why we use acetic acid, which reacts with the sodium hypochlorite to give hypochlorous acid, which then reacts with the alcohol. Experiment 9 – A Greener Oxidation of Alcohols Procedure 1. In a 250 mL Erlenmeyer flask, place 1.5 mL of cyclohexanol and about 3.5 mL of glacial acetic acid. Danger ! ! Glacial acetic acid is corrosive and its vapors are highly irritating. Wear gloves and do not breathe the vapors. 2. Cool the resulting solution in an ice-bath. Add 30 mL of household bleach drop-wise over 5 minutes with swirling. During the addition, ensure that the temperature of the solution does not exceed 50°C. Danger ! 3. After addition, remove the solution from the ice bath. Stir the solution at room temperature for 1 hour. Test the solution every 10 minutes with starch-iodide paper. If the paper does not turn blue-black, the test is negative and the oxidant has been used up. Add more sodium hypochlorite (bleach) about 1 mL at a time until you get a positive test. 4. Add small portions (half the size a pea) of solid sodium thiosulfate (NaS2O3) with stirring to the reaction mixture. This reduces excess hypochlorite. Continue adding NaS2O3 until the starch-iodide test comes out negative. 5. Slowly add 3M sodium hydroxide to neutralize the remaining acetic acid in solution (you can, of course, calculate the volume of base needed to neutralize the acid). Add sodium hydroxide until the solution is basic (red litmus paper turns blue). ! Sodium hypochlorite is corrosive and hazardous to the environment. Wear gloves and do not breathe the vapors. Experiment 9 – A Greener Oxidation of Alcohols 8. Add about 1 g of sodium chloride to the solution to increase the ionic strength. This makes the polar cyclohexanone less soluble in the water, and more likely to dissolve in the organic layer. 9. Pour the solution into a separatory funnel, then rinse the sides of the Erlenmeyer with 5 mL dichloromethane and add the rinse to the separatory funnel. Shake, then drain the dichloromethane layer and set it aside. Extract the aqueous layer with another 5 mL of dichloromethane. Combine the two dichloromethane extractions in an Erlenmeyer flask. Remove residual water from the dichloromethane by adding small portions of anhydrous sodium sulfate until no more clumping occurs. 10. Use gravity filtration with fluted filter paper to separate the drying agent from the dichloromethane/cyclohexanone mixture into a pre-weighed 100 mL beaker (use a beaker, not an Erlenmeyer flask). WARM (do not heat) the beaker on a hot plate set to lowest setting. The last 1 mL or so that does not evaporate is the cyclohexanone; there should be no more dichloromethane smell at the end of the heating. Reweigh the beaker to obtain the mass of cyclohexanone. Experiment 9 – A Greener Oxidation of Alcohols Clean Up and Waste Disposal 1. Dispose of all aqueous waste down the drain. 2. Place your product in the vial provided by your instructor. 3. Wash and dry all glassware and equipment. 4. Return all glassware and equipment to their proper locations. Experiment 9: A Green Oxidation of Alcohols Name Section Partner(s) Volume of cyclohexanol _______________ mL Mass of cyclohexanol _______________ g Mass of product _______________ g Yield _______________ % 1. Write a plausible mechanism for the hypochlorous acid oxidation of cyclohexanol under acidic conditions. Be sure to staple the duplicate pages from your laboratory notebook at the back of this laboratory data sheet before handing in to your instructor.