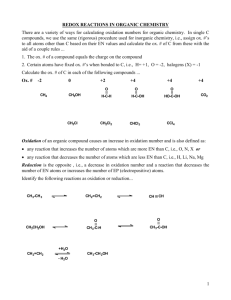
Powerpoint Redox
advertisement
Transfer of electrons Oxidation state Rule number 1 Rule number 2 Rule number 3 Rule number 4 Rule number 5 Rule number 6 Rule number 7 Rule number 8 Let’s practice… Determine the oxidation numbers for the atoms in the compound NaCl. Determine which atom will have a positive or negative oxidation number. Determine the oxidation number for each atom Check whether the oxidation numbers add up to the overall charge Give the oxidation number of sulfur in a sulphate ion Determine which atom will have a positive or negative oxidation number by looking at the electronegativity values. Determine the oxidation number for each atom. Determine the oxidation number of sulfur by using the fact that the oxidation numbers of the atoms must add up to the charge on the molecule Determine the oxidation states of the elements K and Br on both the reactant and product side of the following reaction: 2K + Br2 →2KBr In the product determine which atom will have a positive or negative oxidation number by looking at the electronegativity values Use the valency of the elements in the product to determine the oxidation numbers. Check whether the oxidation numbers add up to the charge on the compound Redox Reactions Reduction-Oxidation Let’s look at an example of this… (2x) Half-reaction The oxidation or reduction reaction part of a redox reaction. It is obtained by considering the change in oxidation states of the individual substances that are involved in the redox reaction Oxidation is the loss of electrons and leads to an increase in oxidation number Reduction is the gain of electrons and leads to a decrease in oxidation number OILRIG Oxidation Is Loss of electrons and Reduction Is Gain.