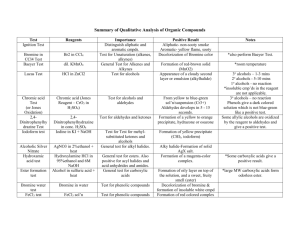
Dess-Martin Oxidation
advertisement

Dess-Martin Oxidation Muhammad asmael Dr.Fuad mahmood contents • • • • • • Objectives Background Reaction and mechanism Application and recent literature Conclusion reference objectives • Dess-Martin periodinane (DMP) is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones background • This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions (room temperature, neutral pH), shorter reaction times, higher yields, simplified workups, high chemoselectivity, tolerance of sensitive functional groups, and a long shelf life. It is named after the American chemists Daniel Benjamin Dess and James Cullen Martin who developed the reagent in 1983. It is based on IBX, but due to the acetate groups attached to the central iodine atom, DMP is much more soluble in organic solvents Reaction and Mechanism Recent Literature Acceleration of the Dess-Martin Oxidation by Water S. D. Meyer, S. L. Schreiber, J. Org. Chem., 1994, 59, 7549-7552. Recyclable 2nd generation ionic liquids as green solvents for the oxidation of alcohols with hypervalent iodine reagents J. S. Yadav, B. V. S. Reddy, A. K. Basak, A. V. Narsaiah, Tetrahedron, 2004, 60, 2131-2135. Application • In one application of the dess-martin oxidation, involves transforming a sensitive α-β-unsaturated alcohol to its corresponding aldehdye. • This moiety has been found in several natural producs and due to its high functionality, it could be a valuable synthetic building block in organic synthesis. conclusion • Iubac name for dess martin oxidation 1,1,1-Triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one • The Baylis–Hillman adducts of aryl aldehydes and alkyl acrylates are efficiently oxidized to the corresponding αmethylene-β-keto esters with the Dess–Martin periodinane. Reference • http://www.organic-chemistry.org/namedreactions/dessmartin-oxidation.shtm • ^ Dess, D. B.; Martin, J. C. (1983). "Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones". J. Org. Chem. 48: 4155. doi:10.1021/jo00170a070. • Boeckman, R. J. In "Encyclopedia of Reagents for Organic Synthesis"; Paquette, L. A., Ed.; Wiley: Chichester, UK, 1995, Vol. 7, pp. 4982-4987. (Review) THANKS FOR ATTENTION