Redox Reactions in Organic Chemistry Worksheet
advertisement
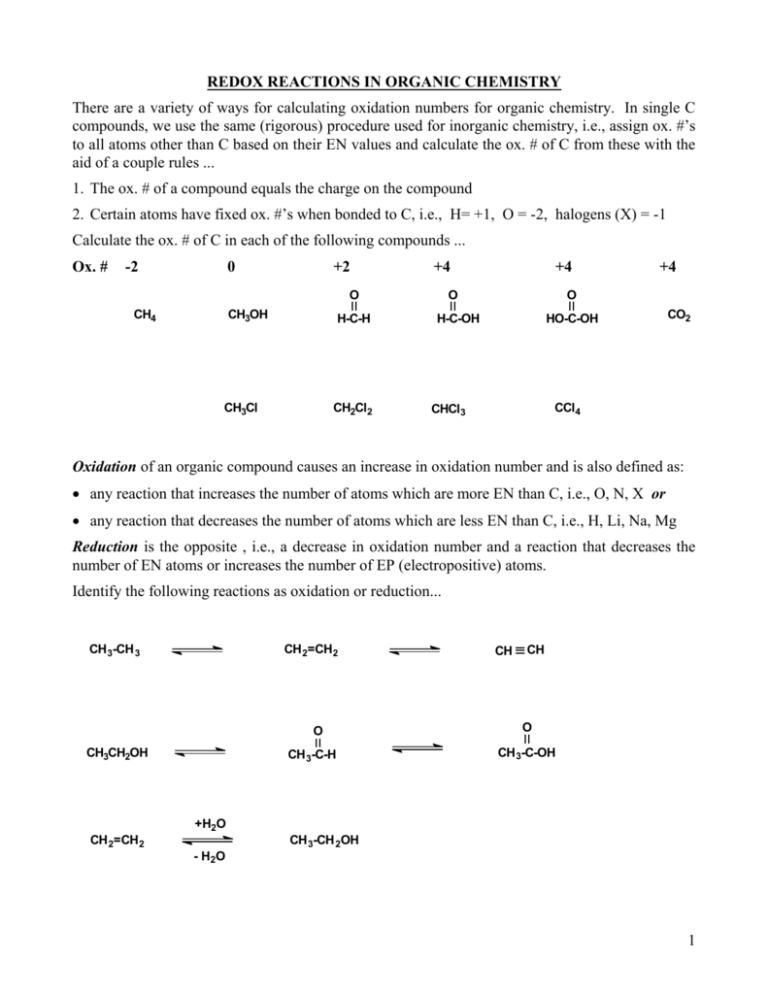
REDOX REACTIONS IN ORGANIC CHEMISTRY There are a variety of ways for calculating oxidation numbers for organic chemistry. In single C compounds, we use the same (rigorous) procedure used for inorganic chemistry, i.e., assign ox. #’s to all atoms other than C based on their EN values and calculate the ox. # of C from these with the aid of a couple rules ... 1. The ox. # of a compound equals the charge on the compound 2. Certain atoms have fixed ox. #’s when bonded to C, i.e., H= +1, O = -2, halogens (X) = -1 Calculate the ox. # of C in each of the following compounds ... Ox. # -2 0 +2 +4 O CH4 CH3OH H-C-H CH3Cl CH2Cl2 +4 O +4 O H-C-OH HO-C-OH CO2 CCl4 CHCl3 Oxidation of an organic compound causes an increase in oxidation number and is also defined as: any reaction that increases the number of atoms which are more EN than C, i.e., O, N, X or any reaction that decreases the number of atoms which are less EN than C, i.e., H, Li, Na, Mg Reduction is the opposite , i.e., a decrease in oxidation number and a reaction that decreases the number of EN atoms or increases the number of EP (electropositive) atoms. Identify the following reactions as oxidation or reduction... CH 3-CH 3 CH 2=CH 2 O CH3CH2OH CH 3-C-H CH CH O CH 3-C-OH +H2O CH 2=CH 2 CH 3-CH 2OH - H 2O 1 A Simpler Method for Calculating Oxidation ‘Levels’ of Organic Compounds: Consider the oxidation in the following compound ... O H CH2 C OH Cl CH2 C H [O] H Cl NO2 NO2 1. Ignore the section of the compound which is unchanged. Calculate the change in oxidation level only for the C’s affected by the reaction and consider only the atoms directly bonded to C. 2. Reverse the calculations ( signs) of the previous method ... a bond to H (or anything EP) makes C = -1 a bond to O (or anything EN) makes C = +1 a bond to another C makes C = 0 Calculate the change in oxidation level in the reaction above. The formula below may be used. (# C-O bonds) - (# C-H bonds) = ox. level or N or X or Li, Mg, etc. Oxidizing Reagents for Organic Chemistry Oxidant (media) Reduced Form Oxidant Reduced Form (purple) MnO4 (OH or neutral) MnO2 (brown ppte.) X2, e.g., Cl2 X-, e.g., Cl- (purple) MnO4- (H+) Mn+2 (colorless, aq.) H2O2 2 O-2 hot conc. HNO3 NO2 (brown gas) O2 2 O-2 (orange) H2CrO4 (aq. H2SO4) Cr+3 (blue-green) Pb(OAc)4 Pb(OAc)2 Hg(OAc)2 HgOAc NaOCl (bleach) Cl- - - PCC (in CH2Cl2) HIO4 Cr +3 (blue-green) HIO3 (colorless) H2CrO4 is prepared by dissolving CrO3 (chromia or chromic anhydride) or K2Cr2O7 in aq. H2SO4. Jones reagent: CrO3 + H2SO4 (aq.) + acetone H2CrO4 Pyridinium Chlorochromate (PCC): CrO3 + C5H5N: + HCl C5H6NCrO3Cl Collins Reagent: CrO3 + 2 C5H5N: (C5H5N)2CrO3 Cr+6 in aqueous acid soln. are strong oxidants. Cr+6 in anhydrous solvent are mild oxidants. 2 Reducing Agents for Organic Chemistry Reducing Reagent (conditions) Oxidized form H2 (g) (Pt, Pd, Ni, etc. catalyst in EtOH) H+ LiAlH4 (in ether) liberates :H- H2 NaBH4 (in EtOH) liberates :H- H2 BH3 (g) H2 Li, Na, K, Zn, Hg, Mg, etc. Li+, Na+, K+, Zn+2, etc. SnCl2 + 2HCl = H2SnCl4 H2SnCl6 Zn[Hg] + HCl ZnCl2 + H2 Balancing Redox Equations: In order to calculate the theoretical and actual yield from a reaction, a balanced chemical equation is necessary. The following rules describe the Ion-Electron Half-Reaction Method for balancing redox equations ... 1. Break the equation into 2 half-reactions, i.e., oxidation and reduction 2. Balance all atoms in each half-reaction other than H and O 3. Balance O and H as follows ... add H2O to balance O’s first, then balance H’s by adding H+ add sufficient electrons to balance the charges Note: e-‘s are always added to the right side of the oxidation ½-reaction because ox. = loss of e-‘s Note: e-‘s are always added to the left side of the reduction ½-reaction because red. = gain of e-‘s if the reaction is carried out in alkaline media, add enough OH- to neutralize all H+ (combine them to make HOH) and be sure to add the same quantity of OH- to both sides to keep mass and charges balanced 4. Multiply each ½-reaction by a least common multiple so the number of e-‘s transferred in each ½-reaction is equal. 5. Add the ½-reactions and cancel common terms from each side 6. Check for charge and mass balance Balance the following redox equations for practice ... 1. Cyclopentanol is cleaved by strong oxidants like hot, conc. HNO3 producing 1,3-dipentanoic acid. 2. Cyclohexene is cleaved by strong oxidants like hot, acidic KMnO4 3. Cyclohexene is oxidized to a diol by cold, neutral or alkaline KMnO4 3