Intermolecular Forces - Montgomery County Schools
advertisement

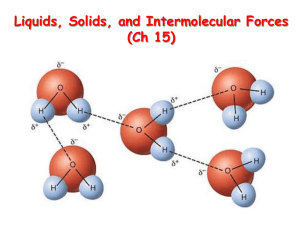
Intermolecular Forces Intra- vs. Inter Intra-: inward – Ex. Intradermal, Intravenous Inter-: between or among – Ex. Interstate, International Intramolecular forces act within a molecule. – Ionic, covalent, and metallic bonding Intermolecular forces act between molecules. – London dispersion, dipole-dipole interactions, ion-dipole interactions, and Hydrogen bonding Intramolecular vs. Intermolecular Forces - Similarities Attractive forces Force due to electron sharing (charge) Affect spatial arrangements of atoms and molecules, respectively Intramolecular vs. Intermolecular Forces - Differences Intramolecular Forces Intermolecular Forces Strong Weak Act within molecules Act between molecules Persist for life of molecule More brief in life of molecule Not strongly effected by physical changes Stabilize individual molecules Strongly effected by physical changes Responsible for bulk properties of matter Lava Lamp Experiment 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Each pair of students should write their names on the top of 1 sheet of paper. One student should obtain a 250 mL beaker and fill halfway with water. A second student should add about 1 cm of oil to top (between ¼ and ½ inch or about the width of pinky finger). On your piece of paper, draw and describe the beaker and label it as “Drawing 1”. When Drawing 1 is complete, raise your hand for addition of 1 drop of food coloring. Draw the beaker and its contents immediately after the addition of food coloring. Label this “Drawing 2”. Wait 1-2 minutes and draw the beaker again. This is “Drawing 3”. When Drawings 2 and 3 are complete, raise your hand for addition of sugar. Draw and describe what happens right after the sugar is added as “Drawing 4”. Draw and describe what happens about one minute after the sugar is added as “Drawing 5”. Lava Lamp Experiment 1. 2. 3. 4. 5. What materials will mix together? What happened to food coloring in oil? How would you describe the color, shape, size, and movement of the food coloring? What happened to food coloring in water? How would you describe the color, shape, size, and movement of the food coloring? What do you think the phrase “like dissolves like” means? How would you apply “like dissolves like” to the materials used in the beaker? Intermolecular Forces London dispersion forces Dipole-dipole forces Hydrogen bonding Ion-dipole forces Name of force Rank of strength Ion involved? Polar or nonpolar molecules? Is H involved? Example Why don’t oil and water mix? London dispersion forces Weakest intermolecular force Only attractive force between non-polar molecules Created from temporary fluctuations in electron density around atoms The larger the molecule, the greater the dispersion force. https://www.chem.unsw.edu.au/coursenotes/CHEM1/nonunipass /hainesIMF/dispersion.html London dispersion forces Why don’t oil and water mix? Lava Lamp Experiment Which materials exhibit London dispersion forces? What observations can be explained by London dispersion forces? Name of force London dispersion forces Rank of strength 4 Ion involved? No Polar or nonpolar molecules? Both (strongest for nonpolar) Is H involved? No Example Oil and water Dipole In polar molecules electrons are not equally shared between atoms. In areas of the electron cloud where electrons are more likely to be found, a “dipole” is formed. This end of the molecular has a partial negative charge. The opposing side of the molecule will have a partial positive charge. These molecules are “polar”. Dipole Cl δ - H δ+ Example: H2O δ δ+ - H O Example: HCl δ - H+ δ Images modified from: http://employees.csbsju.edu/hjakubowski/classes/ch123/ch123ch2mcmfay5th.htm Dipole-Dipole Forces Attractive force between neutral, polar molecules (molecules that possess a dipole). The larger the dipole, the greater the force. Animation Image modified from: http://www.chem.ufl.edu/~itl/2041_u01/lectures/lec_g.html Lava Lamp Experiment What materials exhibit dipole-dipole interactions? What observations can be explained by dipole-dipole interactions? Name of force Rank of strength Ion involved? Polar or nonpolar molecules? Is H involved? Example London dispersion forces 4 No Both (strongest for nonpolar) No Oil and water Dipoledipole forces 3 No Polar No Alcohol in water Why does salt dissolve? Ion-Dipole Forces Interaction between charged molecule (ion) and polar molecule (dipole). Strength depends on charge and size of ion and magnitude and size of dipole – Cations interact more strongly with dipoles than anions. Image from: http://www.chem.purdue.edu/gchelp/liquids/iondip.html Why does salt dissolve? NaCl in Water How would heating affect solubility? Stirring? Lava Lamp Experiment Were there any ion-dipole forces in the lava lamp experiment? Can any observations be explained by ion-dipole forces? Name of force Rank of strength Ion involved? Polar or nonpolar molecules? Is H involved? Example London dispersion forces 4 No Both (strongest for nonpolar) No Oil and water Dipoledipole forces 3 No Polar No Alcohol in water Ion-dipole forces 1 Yes Polar No Salt in water Hydrogen Bonding Permanent A H B Where A and B are F, N, or O dipole-dipole interaction Only occurs in molecules containing H-F, H-N, or H-O bonds Hydrogen Bonding of Water Hydrogen Bonding of Water Snowflakes Hydrogen Bonding & Boiling Point Image from: http://faculty.ycp.edu/~peterman/chm136/chm136s07ex1a.htm Lava Lamp Experiment Were there any hydrogen bonds in the lava lamp experiment? Can any observations be explained by hydrogen bonding? Name of force Rank of strength Ion involved? Polar or nonpolar molecules? Is H involved? Example London dispersion forces 4 No Both (strongest for nonpolar) No Oil and water Dipoledipole forces 3 No Polar No Alcohol in water Ion-dipole forces 1 Yes Polar No Salt in water Hydrogen bonding 2 No Polar Yes Water “Like dissolves like” To be soluble a compound must interact with the solute by: – Dipole-dipole forces – Ion-dipole forces – London dispersion forces Polar solutes dissolve in polar solutions Non-polar solutes dissolve in nonpolar solutions Summary Image from: http://www.chem.ufl.edu/~itl/2041_u01/lectures/lec_g.html