History of Atomic Theories
advertisement
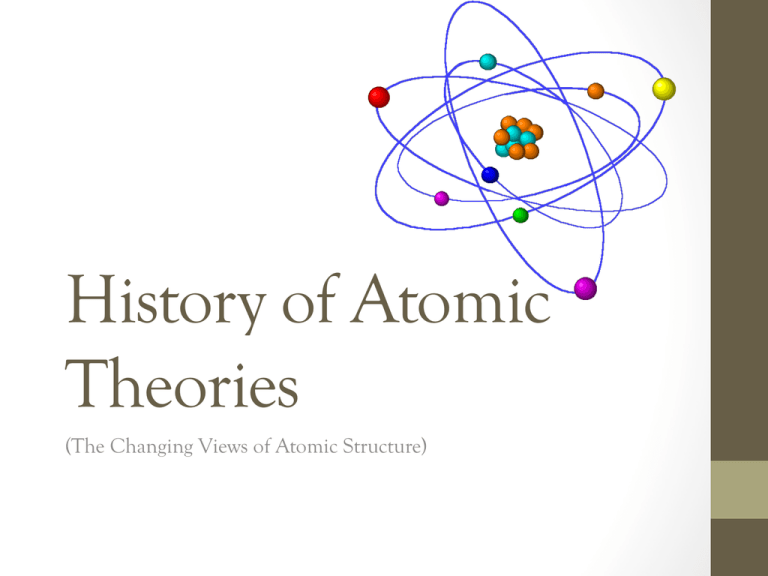
History of Atomic Theories (The Changing Views of Atomic Structure) Empedocles (Greek Philosopher, 450 B.C.) Everything that existed was thought to be composed of four elements. (i) Water (iii) Air (ii) Fire (iv) Earth Democritus (Greek Philosopher, 400 B.C.) All matter is made of tiny hard indestructible particles which are indivisible (i.e. can not be broken down). The Greek word for “indivisible” is atomos, therefore all matter is made up of atoms. Aristotle (Greek Philosopher, 350 B.C.) Believed in Empedocles’ “four element” model. -Despite the more recent “atomic model” proposed by Democritus, Aristotle was so influential that the “four element” model was accepted for almost 2000 years. Note: The Greek philosophers did not test their ideas with experiments. They were thought of as great “thinkers”, but not scientists. Alchemists (Philosophers, Mystics, Magicians, Chemists, 500-1600 A.D.) Performed experiments and devised chemical symbols for substances that we now call elements and compounds. They also invented many lab tools that are still used today… John Dalton (English Scientist, 1800’s) Using experiments, he discovered that 1. Atoms are tiny indestructible particles that cannot be broken down 2. Atoms combine with other atoms to form molecules (e.g. carbon + oxygen carbon dioxide) 3. Atoms of an element are identical (e.g. H2 gas are all H atoms) 4. Atoms do not lose their identity during chemical reactions (i.e. cannot be created and/or destroyed) 5. Molecules of a compound are identical (e.g. water = H2O) Dalton developed a 6 part theory based on these experiments which explains 1. The Law of Conservation of Mass The total mass of the reactants = the total mass of the products 2. The Law of Constant Composition A compound always contains the same elements in the same proportions by mass. Model: Billiard Balls Theory Model Analogy (Featureless Sphere) (Billiard Balls) Indivisible atoms J.J. Thompson (Physicist, 1904) Thompson discovered electrical particles can be lost or gained from elements. He named these particles electrons. He also believed that atoms were made of positively charged matter with negatively charged electrons scattered throughout. Model: The Raisin Bun Model Theory Model Analogy (Uniform Charge Distribution) (Raisin Bun) Electrons embedded within a positive sphere Net charge of zero Hantaro Nagaoka (Japanese Scientist, 1904) He modeled the atom as a large positive sphere surrounded by a ring of negative electrons. Model: The Saturn Model Theory Model Analogy (Ring of Flat Electrons) (Saturn) Positive sphere with ring of electrons Ernest Rutherford (Nuclear Physicist, 1911) At McGill University (in Montreal), Rutherford designed the “Gold Foil Experiment” to test Thompson’s and Nagaoka’s models The Gold Foil Experiment Positive alpha particles were fired at a thin sheet of gold foil Rutherford predicted that the alpha particles would pass directly through the metal foil, untouched. The Gold Foil Experiment The Gold Foil Experiment The Results… Most alpha particles passed through in a direct path, however, some deflected and bounced back. “Its like shooting a bullet at a piece of tissue paper and having the bullet bounce off” The Gold Foil Experiment The Conclusion… Atoms are mostly empty space with a tiny central nucleus which contains almost all of the total mass of the atom and is positively charged. He called these positively charged particles protons. Protons have a mass nearly 200x greater than electrons. The nucleus is surrounded mostly by empty space, containing rapidly moving negative charges called electrons. Model: The Nuclear Model Theory Model Analogy (Nuclear Model) (Beehive) Small positive nucleus surrounded by negative electrons James Chadwick (Nuclear Physicist, 1932) Chadwick discovered particles in the nucleus of an atom possessing no electrical charge (neutral). He called them neutrons. The neutrons make up the remaining mass of the nucleus. Neils Bohr (Danish Physicist, 1920) Suggested electrons moved around the nucleus in a definite “orbit” arranged in “shells”. Model: The Planetary Model Theory Model Analogy (Planetary Model) (Planets Orbiting Around the Sun) Explains periodic law Electrons are quantized in energy levels Neutrons + Protons are in the nucleus Electrons in orbits around the nucleus Protons = Positive Charge Electrons = Negative Charge Neutrons = Zero Charge HOMEWORK Read pg. 82 – 85 Answer # 1 – 6, 9 on pg. 85 Read pg. 90 – 92 Answer # 1 – 3 on pg. 93 The End










