(Atomic Theory) Class Activity/Notes
advertisement

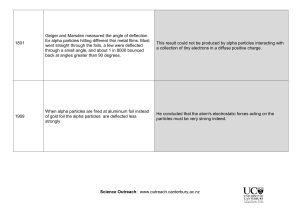
Chapter 5 (Atomic Theory) Class Activity/Notes 1. State the main ideas of Dalton’s atomic theory. Atoms are Tiny. Atoms of the same element are the same. Atoms of different elements are different. Atoms can’t be divided, created or destroyed. Atoms of different elements combine in simple whole-number ratios to form compounds. 2. Characterize the size of an atom. Atoms are extremely tiny. Individual atoms cannot be seen even with a microscope. 10 atoms would be about a nanometer (1 billionth of a meter) Copper has about 100 000 000 atoms for every centimeter. 3. Democritus and Dalton both proposed that matter consists of atoms. How did their approaches to reaching that conclusion differ? Democritus lacked the experimental support that would have helped his argument. Scientific testing was unknown during his time. His theory also did not explain chemical behavior. Dalton used experimental evidence to support his theory. He found that when elements combined to make compounds they always combined in simple wholenumber ratios according to their masses. 4. What are the charges and relative masses of the three main subatomic particles? Subatomic Particle Charge Mass Location Proton 1+ 1 amu nucleus Neutron 0 1 amu Electron 1- 1/1840 amu nucleus (electron cloud) large distance outside nucleus 5. Describe the basic structure of an atom. Atoms consist of a nucleus containing the protons and neutrons. Electrons are found in various locations at relatively large distances away from the nucleus. The nucleus is difficult to get at because it is shielded by the outer electrons so when elements react with other elements it usually results in changes to the electrons only. 6. Describe Thomson’s, Millikan’s, and Rutherford’s contributions to atomic theory. Include their experiments if appropriate. J.J. Thomson (1897): Discovered the electron by working with the cathode ray tube (Crooke’s tube). Cathode rays were deflected by a negative field so they were negatively charged particles. Thomson Revised Dalton’s model by showing equal amounts of positive (+) and negative (-) charges distributed throughout the atom. Thomson named his model of the atom the “Plum Pudding Model” after a common dessert Robert A. Millikan (1868-1953) Carried out experiments (Oil drop experiment) that allowed him to find the quantity of charge carried by an electron. From the oil-drop experiment he was able to determine the relative charge to mass ratio of an electron. Millikan determined the mass of an electron was 1/1840 or 2000 times smaller than the mass of a single hydrogen atom. Ernest Rutherford (Gold Foil Experiment): (1909) The discovery of a “nucleus” Known for his famous “Gold foil experiment” Rutherford gave us the atom with a dense positive nucleus and electrons found outside the nucleus. (+) alpha radiation Radioactive source The Gold Foil Experiment: The set up: Use a radioactive source (Polonium) that emits alpha particles (+ charged helium ions). Shoot the alpha (+) particles at a thin layer of Gold foil. Use a screen coated with phosphorus surrounding the Gold foil to see where the alpha particles go. The results: Most of the alpha particles went straight through the Gold foil. Some of the alpha particles were deflected to the sides. A few of the alpha particles bounced straight back. The conclusions: The atom is mostly empty space. The mass of the atom is located in a dense central core called the nucleus. Evidence to support conclusion (Most particles went straight through) (A few particles bounced back) The particles deflected or bounced back. The nucleus has all the positive charge. Electrons must be in motion around the nucleus since they do not crash into the nucleus (Like charges repel each other. (+) alpha particles were repelled by the nucleus) (Most particles went straight through)