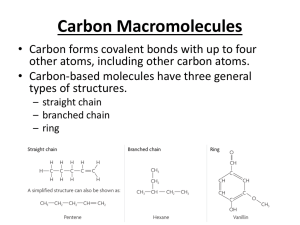
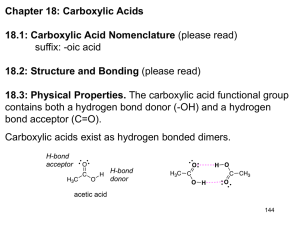
Organic Chemistry II Introduction
advertisement

Organic Chemistry II Carboxylic Acid Derivatives Dr. Ralph C. Gatrone Department of Chemistry and Physics Virginia State University Spring, 2011 1 Chapter Objectives • Nomenclature • Properties • Preparation • Reactions • Spectroscopy Spring, 2011 2 Carboxylic Derivatives • Functional Groups we will consider are: Spring, 2011 3 Carboxylic Derivatives • Chemistry is similar • Chemistry is dominated by one reaction O R Spring, 2011 O Nu: X R Nu 4 Nomenclature Acid Halides RCOX • Derived from the carboxylic acid name by replacing the -ic acid ending with -yl or the carboxylic acid ending with –carbonyl and specifying the halide Spring, 2011 5 Nomenclature Acid Anhydrides, RCO2COR’ • If symmetrical replace “acid” with “anhydride” based on the related carboxylic acid • • For substituted monocarboxylic acids: use bis- ahead of the acid name • Unsymmetrical anhydrides— cite the two acids alphabetically Spring, 2011 6 Nomenclature Esters, RCO2R • Name R’ and then, after a space, the carboxylic acid (RCOOH), with the “-ic acid” ending replaced by “-ate” Spring, 2011 7 Nomenclature Amides, RCONH2 • With unsubstituted NH2 group. replace -oic acid or -ic acid with -amide, or by replacing the -carboxylic acid ending with –carboxamide • If the N is further substituted, identify the substituent groups (preceded by “N”) and then the parent amide Spring, 2011 8 Examples O • Try these O(CH2)7 CH3 O CH3COCH3 O O Br Br O Cl O O N Spring, 2011 9 Examples • Answers O O(CH2)7CH3 octyl crotonate O CH3COCH3 O O Br methyl acetate Br O Cl bis-bromoacetic anhydride pentanoyl chloride O O N Spring, 2011 N-ethyl-N-isopropylacrylamide 10 Properties • Amides have higher boiling point than other derivatives due to H-bonding • Esters have pleasant odors associated with fruits and flavors • Acid halides have pungent odors due to hydrolysis to HCl or HBr Spring, 2011 11 Stability • • • • Amides more stable than Esters, more stable than Acid anhydrides, more stable than Acid halides • Esters and amides are commonly found in • • natural materials. Amides make up foundation of animals Acid halides and anhydrides don’t exist in nature Spring, 2011 12 Stability Spring, 2011 13 Relative Reactivity of Carboxylic Acid Derivatives • Nucleophiles react more readily with unhindered carbonyl groups • More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) • The intermediate with the best leaving group decomposes fastest Spring, 2011 14 General Reactions of Carboxylic Acid Derivatives Spring, 2011 15 Reactions of Carboxylic Acids • OH is poor leaving group • Must enhance reactivity • Specific reagents can produce acid chlorides, anhydrides, esters, amides Spring, 2011 16 Acids to Acid Chlorides • Reaction with thionyl chloride, SOCl2 Spring, 2011 17 Acids to Acid Anhydrides • Heat cyclic dicarboxylic acids that can form five• or six-membered rings Acyclic anhydrides are not generally formed this way - they are usually made from acid chlorides and carboxylic acids Spring, 2011 18 Acids to Esters • Carboxylate anion with a primary alkyl halide Spring, 2011 19 Fischer Esterification • Heating a carboxylic acid in an alcohol solvent containing a small amount of strong acid produces an ester from the alcohol and acid Spring, 2011 20 Esterification using Diazomethane CH2N2 O O ETHER CO2 H CO2 CH3 Reaction only provides the methyl ester Glassware must be polished (no ground glass joints) Spring, 2011 21 Acids to Amides • Direct reaction cannot be done base CO2H acid (CH3)2NH CO2 + (CH3)2NH2 salt • Due to acid base reaction that occurs first Spring, 2011 22 Acids to Amides • Must make OH a better leaving group • Generally done in the laboratory via the acyl halide • Can also use dicyclohexylcarbodiimide to activate the OH O R O O R N Spring, 2011 C HN O O N RNH2 R NHR N 23 Chemistry of Acid Halides • Acid chlorides are prepared from carboxylic acids • by reaction with SOCl2 Reaction of a carboxylic acid with PBr3 yields the acid bromide Spring, 2011 24 Reactions of Acid Halides • • • • Nucleophilic acyl substitution Halogen replaced by OH, by OR, or by NH2 Reduction yields a primary alcohol Grignard reagent yields a tertiary alcohol Spring, 2011 25 Acid Halides into Acids (Hydrolysis) • Acid chlorides react with water to yield • carboxylic acids HCl is generated during the hydrolysis: a base is added to remove the HCl Spring, 2011 26 Acid Halides to Anhydrides • Acid halides react with carboxylate anions O Cl Spring, 2011 CH3CH2C O O O O O CH2CH3 27 Acid Halides to Esters • Esters are produced in the reaction of acid • chlorides react with alcohols in the presence of pyridine or NaOH The reaction is better with less steric bulk Spring, 2011 28 Acid Halides into Amides • Amides result from the reaction of acid chlorides – with NH3, – primary amines (RNH2) – secondary amines (R2NH) • The reaction with tertiary amines (R3N) gives an unstable species that cannot be isolated • HCl is neutralized by the amine or an added base Spring, 2011 29 Acid Chlorides into Alcohols • LiAlH4 reduces acid chlorides to yield primary alcohols through the aldehyde (not isolated) Spring, 2011 30 Acid Halides with Organometallics • Grignard reagents react with acid chlorides to yield tertiary alcohols in which two of the substituents are the same Spring, 2011 31 Acid Chlorides to Ketones • Reaction of an acid chloride with a lithium diorganocopper (Gilman) reagent, Li+ R2Cu • Reaction of an acid chloride with organocadmium reagent O R Spring, 2011 O R2Cd Cl R R 32 Chemistry of Acid Anhydrides • Prepared by carboxylate nucleophile with an acid chloride Spring, 2011 33 Reactions of Acid Anhydrides • Similar to acid chlorides in reactivity Spring, 2011 34 Anhydrides to Esters or Amides • Anhydrides form esters with alcohols • Anhydrides form amides with amines Spring, 2011 35 Anhydrides: More Selective than Acid Halides • Anhydrides react with amines in presence of alcohols, Not so acid chlorides O O NH2 NH2 N H Cl HO HO H N NH2 HO O O O O O O O NH2 HO Spring, 2011 O N H O HO 36 Chemistry of Esters • Many esters are pleasant-smelling liquids: • fragrant odors of fruits and flowers Also present in fats and vegetable oils Spring, 2011 37 Preparation of Esters • Esters are usually prepared from carboxylic acids Spring, 2011 38 Reactions of Esters • Less reactive toward nucleophiles than are acid • chlorides or anhydrides Cyclic esters are called lactones and react similarly to acyclic esters Spring, 2011 39 Esters into Carboxylic Acids • An ester is hydrolyzed by aqueous base or aqueous acid to yield a carboxylic acid plus an alcohol Spring, 2011 40 Soap • Ester (Fat) hydrolysis into carboxylate anion O R O O O R O NaOH R O O R O - Na+ OH HO OH soap R = C11 to C17 hydrocarbon triglyceride (animal fat) Spring, 2011 41 Esters to Amides • Ammonia reacts with esters to form amides • Reaction is easier from the acid chloride Spring, 2011 42 Esters into Alcohols: Reduction • Reaction with LiAlH4 yields primary alcohols Spring, 2011 43 Reduction of Esters • • • • • LAH is reducing agent of choice NaBH4 does not reduce esters H2 does not reduce esters BH3 – THF does not reduce esters DIBAH reduces esters to the aldehyde O O OMe DIBAH H toluene DIBAH = Spring, 2011 Al H 44 Esters with Grignard Reagents • Reacts with 2 equivalents of a Grignard reagent to yield a tertiary alcohol Spring, 2011 45 Chemistry of Amides • Prepared by reaction of an acid chloride with • ammonia, primary amines, or secondary amines Amides are the least reactive of derivatives Spring, 2011 46 Reactions of Amides • Heating in either aqueous acid or aqueous base produces a carboxylic acid and the amine Spring, 2011 47 Amides into Amines • Reduced by LiAlH4 to an amine rather than an • alcohol Converts C=O CH2 Spring, 2011 48 Reduction of Amides • Works with cyclic and acyclic • Good route to cyclic amines Spring, 2011 49 Amides to Nitriles O CN NH2 SOCl2 or POCl3 can be used Spring, 2011 50 Amide to Amine The Hoffmann Rearrangement • Preparation of amine with loss of a carbon O NH2 Spring, 2011 Br2/NaOH/H2O NH2 + CO2 51 Infrared Spectroscopy • Acid chlorides: 1800cm-1 • Acid anhydrides: 1820 and 1760cm-1 • Esters: 1735cm-1 • Amides: 1650 - 1690cm-1 depending on N substitution Spring, 2011 52 NMR Spectroscopy • Carbon-13 NMR • Difficult to assign with certainty Spring, 2011 53 NMR Spectroscopy • Proton NMR • Protons adjacent to C=O resonate near 2d • Identity of the C=O cannot be determined Spring, 2011 54