Carbohydrates
advertisement
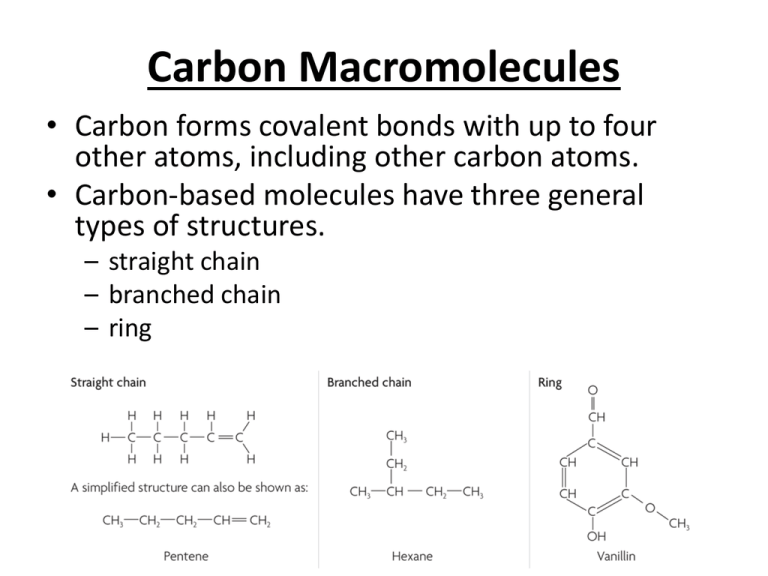
Carbon Macromolecules • Carbon forms covalent bonds with up to four other atoms, including other carbon atoms. • Carbon-based molecules have three general types of structures. – straight chain – branched chain – ring • Many carbon-based molecules are made of many small subunits bonded together. – Monomers are the individual subunits. – Polymers are made of many monomers. AKA: Hydrocarbons Alkanes C C H H H H H C C C C C H H H H H C C H Alkynes Alkenes H H C C H H H C C C C C H H H H H Aromatics H H C C H H H C C C H H H H H H H C C C C H C C H H Hydroxyl, carbonyl, carboxyl • There are other names that describe patterns of atoms that are parts of functional groups: • “Hydroxyl” refers to –OH • “Carbonyl” refers to C=O • “Carboxyl” refers to COOH Q: Which functional groups contain a hydroxyl group? A carbonyl group? A carboxyl group? Hydroxyl: alcohols, carboxylic acids. Carbonyl: aldehydes, ketones, carboxylic acids, amides, esters. Carboxyl: carboxylic acids *Note that properties such as boiling and melting point change due to functional groups Carbohydrates •Function: To be broken down as a source of chemical energy; also a part of cell structure. •Example: sugars, starches, cellulose *Simple sugars (glucose) = monosaccharides *Starches and cellulose = polysaccharides Importance of polysaccarides: • Plants store glucose in the form of polysaccharides known as starch in their roots • Animals store glucose in the from of a polysaccharide known as glycogen in our liver and muscle cells • The chains sticking out of the proteins in the cell membrane are polysaccharides know as cell markers • Cellulose is the most abundant organic molecule on earth. – Gives trees and plants structure and strength – We need cellulose (fiber) to keep our digestive tracts clean and healthy • Polysaccharides are used in the shell of crustaceans like crabs and lobsters (Chitin) http://rasamalaysia.com/uploaded_images/black_pepper_crab/stone_crab1.jpg http://chemistry2.csudh.edu/rpendarvis/1feb23.gif http://kentsimmons.uwinnip eg.ca/cm1504/Image70.gif Glucose can be linear or ring structure! Sucrose = glucose + fructose Carbs +/- Water • Dehydration Synthesis vs. Hydrolysis Taking out water to make some thing new. Water breaks down a complex molecule into a simple molecule. Lipids • Function: To be broken down as a source of chemical energy; makes cell membranes • Example: fats, oils, cholesterol *Lipids are nonpolar/hydrophobic! *Most membrane lipids (phospolipids) are amphipathic, having a non-polar end and a polar end. Fatty acids – Chains of carbon atoms bonded to hydrogen atoms (carboxylic acids) Various Fatty Acids: Saturated fats (usually from animals) • • • • single carbon bonds Strong attractions between chains High melting points Solids at room temperature Unsaturated fats (usually from plants) • • • • at least 1 carbon double bond Few interactions between chains Low melting points Liquids at room temperature http://twistedphysics.typepad.com/.a/6a00d8341c9c1053ef0133ecf8b451970b-pi Your Body Needs Lipids! =Tryglyceride Properties of Lipids Hydrogenation • Unsaturated compounds react with H2 • Ni or Pt catalyst • C=C bonds C–C bonds Hydrolysis • Split by water and acid or enzyme catalyst • Produce glycerol and 3 fatty acids 15 Proteins • Function: Many functions- including movement, transport, chemical catalysts • Example: enzymes, meats, nuts Protein- Polymer made of monomers called amino acids. http://www.accessexcellence.org/RC/VL/GG/ecb/ecb_images/04_02_polypeptide_backbone.jpg Nucleic Acids • Function: To store genetic information and build proteins (just ONE function!) • Example: DNA and RNA Nucleic acids- Polymers that are made up of monomers called nucleotides. http://wiki.chemeddl.org/images/c/c8/Chapter_20_page_27-2.jpg http://www.stanford.edu/group/pandegroup/fold ing/education/dna.gif