A. Acid Halides
advertisement
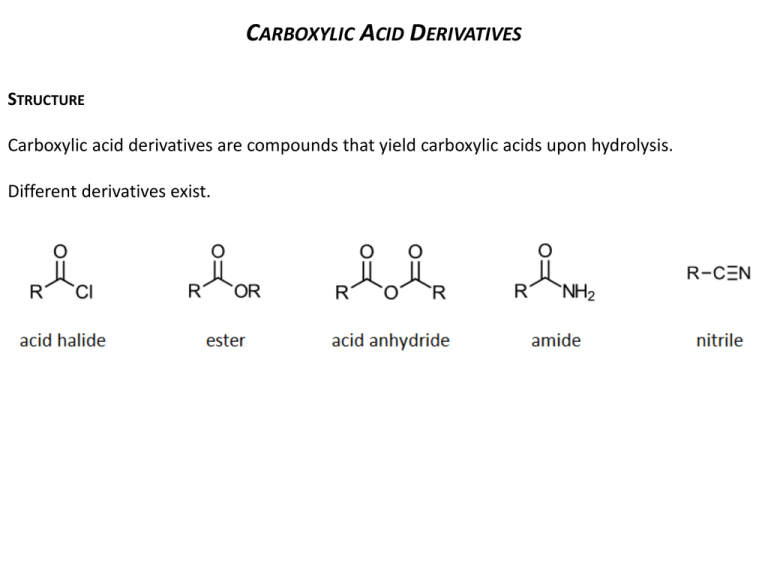
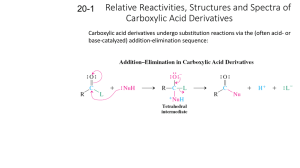
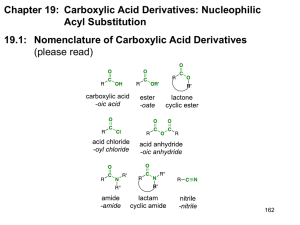
CARBOXYLIC ACID DERIVATIVES STRUCTURE Carboxylic acid derivatives are compounds that yield carboxylic acids upon hydrolysis. Different derivatives exist. NOMENCLATURE Their names are derived from the carboxylic acid names. A. Acid Halides Change the suffix -ic acid to -yl halide. Change the suffix -carboxylic acid to -carbonyl halide. B. Esters Name the alkyl group attached to oxygen first and then (after a space) name the acyl group by changing the -ic acid to -ate. Those attached to rings are named using the suffix -carboxylate. C. Acid Anhydrides If symmetrical, change the acid ending of the carboxylic acid to the word anhydride. When the groups are different, list the names of the two acids alphabetically and add the word anhydride. D. Amides With unsubstituted -NH2 group, drop –ic acid or -oic acid from the name of the parent acid and add -amide, or by replacing the -carboxylic acid ending with -carboxamide If the N is further substituted, identify the substituent groups (preceded by “N”) and then the parent amide. E. Nitriles Add the suffix -nitrile to the name of the parent hydrocarbon chain (including the triply bonded carbon of CN). Replace the -ic acid or -oic acid name of the corresponding carboxylic acid by -onitrile. Carboxylic acid substituents attached to rings are named using the suffix -carbonitrile. Name as an alkyl cyanide (functional class name). PHYSICAL PROPERTIES Boiling points Acid halides, acid anhydrides and esters of same MM have roughly same BP, affected by polarity of carbonyl group, no H-bonding. Amides have strong H-bonding; they have higher BP. Solubility in water They form H-bonds with water. Acid halides, acid anhydrides and esters up to 4-5 carbons are quite soluble. Amides are more soluble up to 6-7 carbons; have extensive H-bonds with H2O. Most low MM acid halides and anhydrides decompose quite rapidly when mixed with H2O. Odours Many esters have pleasant odours, and they are widely used as flavours and fragrances. REACTIVITY Carboxylic acid derivatives react with nucleophiles because they contain an electrophilic unhindered carbonyl carbon. Substitution occurs, not addition, because they have a leaving group on the carbonyl carbon. The order of reactivity depends on the basicity of the substituent attached to the acyl group. Weak base is better at withdrawing electrons inductively from the carbonyl carbon; rendering the carbonyl carbon extremely electrophilic (more susceptible to nucleophilic attack). The weaker the basicity of the substituent attached to the acyl group, the less the carboxylic acid derivative is stabilized by electron delocalization, the more reactive it will be. Weak bases are easier to eliminate (good leaving groups). Acid halides are the most reactive and amides are the least reactive. A result of inductive and resonance effects. inductive effects are not significant A carboxylic acid derivative can be converted into a less reactive carboxylic acid derivative, but not into one that is more reactive. When a nucleophile attacks a carboxylic acid derivative, a reaction can occur in which the nucleophile replaces the leaving group: a nucleophilic acyl substitution. If the nucleophile is negatively charged, it attacks the carbonyl carbon, forming a tetrahedral intermediate. When the tetrahedral intermediate collapses, the weaker base is eliminated. If the nucleophile is neutral, the mechanism has an additional step, the removal of a proton from the tetrahedral intermediate. ACID HALIDES A. Preparation Acid chlorides can be prepared from carboxylic acids and thionyl chloride. Less frequently, PCl3 or PCl5 are used. Acid bromides can be synthesized by using phosphorus tribromide. B. Reactions Acid chlorides are precursors for most of the other acid derivatives. HCl is usually formed as a by-product. A weak base like pyridine (C5H5N) is added to the reaction mixture to remove the strong acid (HCl), forming an ammonium salt. ACID ANHYDRIDES A. Preparation Usually made from acid chlorides and carboxylic acids. The base pyridine (C5H5N) is added to neutralize the HCl formed during the reaction which may react with the anhydride product Sodium salts of carboxylic acids may be used instead of the carboxylic acids. No pyridine is necessary since HCl is not formed. Heat cyclic dicarboxylic acids to form five- or six-membered rings (cyclic anhydrides). Water is eliminated and the ring is formed. B. Reactions Generally analogous to the reactions of acid chlorides. Reactions are usually slower. A carboxylate ion is the leaving group, producing a carboxylic acid (instead of chloride ion). ESTERS A. Preparation Acid chlorides react with alcohols in the presence of pyridine or NaOH to give esters in good yield. Mechanism is nucleophilic addition of the alcohol to the carbonyl as chloride ion leaves, then deprotonation. Fischer esterification: treatment of a carboxylic acid with an alcohol in the presence of an acid catalyst. The reaction is an equilibrium, so it is driven to the right by using excess alcohol or by removing water as it is formed. Mechanism of the Fischer esterification: B. Reactions Esters are hydrolyzed with water in the presence of either acid or base to form carboxylic acids or carboxylate anions, respectively. An ester reacts with an alcohol to form a new ester and a new alcohol. This particular alcoholysis reaction is also called a transesterification reaction because one ester is converted to another ester (useful in converting liquid ester to solid ester; for identification by MP methods). Esters also react with amines to form amides, using ammonia, 1° or 2° amines: aminolysis. Reduction to primary alcohols. Esters react with 2 equivalents of a Grignard reagent to yield a 3° alcohols; 2 step reaction. AMIDES A. Preparation Usually from the reaction of acid chlorides with NH3, primary (RNH2) and secondary amines (R2NH). Reaction with tertiary amines (R3N) gives unstable species that cannot be isolated. A base (NaOH) is added to remove HCl by-product. B. Reactions Nucleophilic acyl substitution reactions proceed slowly. Need a good nucleophile and a catalyst. Hydrolysis is the most common type. Mechanism: acid-catalyzed hydrolysis of an amide to a carboxylic acid Mechanism: base-promoted hydrolysis of an amide to a carboxylic acid Dehydration: primary amides may be "dehydrated" to nitriles (cyano cpds) using phosphorus pentoxide (P2O5 or P4O10) SPECTROSCOPY A. Infrared spectroscopy B. 1H NMR Protons on the carbon to the carbonyl absorb at 2-2.5 ppm. All acid derivatives absorb in the same range so PMR does not distinguish them from each other. The chemical shift of an amide N-H proton is typically between 5-8 ppm. It is broad and often not observed. H H O C C H O H H C C H H d= 4.1 q, J=7.2 Hz, 2H H d= 2.0 s, 3H d= 1.2 t, J=7.2 Hz, 3H O H 3C C N C H 2C H 3 H d 3.4 2H, q, J= 7.0 NH d 2.0 3H, s d 1.1 3H, t, J= 7.0 C. 13C NMR Useful for determining the presence or absence of a carbonyl group. Carbonyl carbon of the various acid derivatives absorb from 160 to 180 ppm. Nitriles give a peak at 115-120 ppm in their 13C NMR spectrum due to the sp hybridized carbon.