3. Pharmacokinetics1 Absorption
advertisement

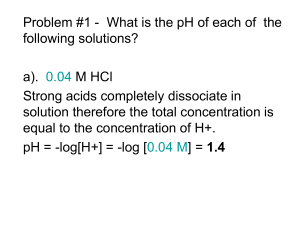
Asmah Nasser, M.D. PHARMACOKINETICS Pharmacokinetics Pharmacokinetics is the quantitative study of drug movement in, through and out of the body What Happens After Drug Administration? Absorption Distribution Metabolism Elimination /Excretion Important factors to know… • Factors affecting absorption • Concept of ionization , pKa and pH • Henderson –Hassel Balch equation • Bioavailability , Bioequivalence , AUC • Distribution , volume of distribution (Vd), redistribution • Plasma protein binding Important factors to know… • Metabolism (biotransformation) , CYP 450 etc • Excretion • First and zero order kinetics of elimination • Plasma Half life • Clearance • Steady state ,loading and maintenance dose • Therapeutic drug monitoring • Pharmacokinetic calculations Absorption After a drug is administered, how does it reaches site of action? Absorption… is the transfer of a drug from its site of administration to the blood stream. In order to reach their site of action, a drug has to pass through several membranes Transport A. Passive diffusion: Concentration gradient across membrane is the driving force for movement of drug molecule across the membrane 1. Small size, water soluble drug molecule penetrate through aqueous channels or paracellular spaces …Filtration …few drugs does this 2. Lipid soluble drugs readily pass through membrane by dissolving in membrane ….many drugs does this B. Active Transport: • Movement occurs against the concentration gradient and needs energy like ATP Factors that affects absorption • The particle size – Smaller is better • concentration gradient • Surface area and vascularity of that area • Lipid solubility / Water solubility • Nature of the drug…acidic or basic • Ionized or non ionized • The pH levels on either side of cell membrane • pKa of the drug Lipid/Water solubility Once in solution, drugs exist as a mixture of two interchangeable forms 1. Water-soluble is the ionized or electrically charged form…Cannot cross membrane 2. Lipid-soluble is the non ionized, or uncharged form….Can cross membrane Concept of ionization…continued • We know most of the drugs are either weak acid or a weak base and when dissolved in body fluids, some or all of a drugs molecules become ionized/unionized HA BH+ H+ + AB + H+ 10 Concept of ionization…continued What percentage is ionized /unionized determined by following factors : 1.Whether the drug was an acid or a base. 2. Whether it is dissolved in an acid or base medium (Eg: Stomach or intestine ?) i.e pH of the medium. 3. And also the pKa of the drug. 11 Role of pH in ionization of a Weak base As pH increases, a weak base will become more and more unionized, lipid soluble and better absorbed As pH decreases, a weak base will become more and more ionized, lipid insoluble, and will not be absorbed. Also becomes more water soluble and better excreted. 12 % of Unionized form Role of pH in ionization of a Weak base 100% 75% 50% 25% 0% 1 pH of the medium 14 13 Role of pH on ionization of a Weak Acid As pH increases, a weak acid will become more and more ionized , lipid insoluble and will not be absorbed. Also becomes more water soluble and better excreted . As pH decreases, a weak acid will become more and more unionized , lipid soluble and better absorbed . 14 % of Unionized form Role of pH in ionization of a Weak acid 100% 75% 50% 25% 0% 1 pH of the medium 14 15 Question Aspirin is an acidic drug. In the stomach, is it mostly in the ionized or unionized form? Moral of the story... Acidic drugs are Absorbed best in Acidic environments Basic drugs are Best absorbed in Basic environments 17 So... To absorption of an basic drug… acidify the environment To absorption of an acidic drug… alkalanize the environment... This concept is very important in treatment of a drug poisoning 18 pKa pKa is equivalent to the pH at which 50% drug is ionized and 50% is unionized….Remember this point pKa Is the negative logarithm of acidic disassociation constant of the weak electrolyte …not necessary to know 19 % of Unionized form 100% 75% 50% 25% 0% 1 14 pH of the medium pKa 20 Henderson-Hasselbalch equation The ratio of lipid-soluble form to water-soluble form for a weak acid or weak base is expressed by the Henderson-Hasselbalch equation The Henderson-Hasselbalch equation relates the ratio of protonated to unprotonated weak acid or weak base to the molecule's pKa and the pH of the medium as follows: 21 Henderson-Hasselbalch equation pH = pKa + log (Unprotonated form) (Protonated form) • For a weak acid, if pH – pKa is low it will be more non-ionized and better absorbed • For a weak base if pH – pKa value is high it will be more non-ionized and better absorbed pH-pKa -2 -1 0 +1 +2 Weak acid: %nonionized 99 90 50 10 01 Weak base: %nonionized 01 10 50 90 99 23 Question 1. The pKa of acetylsalicylic acid is 3.5. What percentage of the drug is in an absorbable form in the stomach at pH of 1.5? A. 0.1% B. 1% C. 10% D. 90% E. 99 Question The greater proportion of the dose of a drug administered orally will be absorbed in the small intestine. However, on the assumption that passive transport of the nonionized form of a drug determines its rate of absorption, which of the following compounds will be absorbed to the least extent in the stomach? a. Ampicillin (pKa = 2.5) b. Aspirin (pKa = 3.0) c. Warfarin (pKa = 5.0) d. Phenobarbital (pKa = 7.4) e. Propranolol (pKa = 9.4) Summary • A drug molecule carrying an electrical charge is said • • • • to be Ionized Lipid-soluble is the non ionized, or uncharged form….Can cross membrane Percentage of ionized drug depends on : 1.Whether the drug was an acid or a base 2. Whether it is dissolved in an acid or base medium (Stomach or intestine ?) i.e pH 3. And also the pKa of the drug As pH increases, a weak base will become more and more unionized ,lipid soluble and better absorbed As pH decreases, a weak acid will become more and more unionized ,lipid soluble and better absorbed Summary pKa is equivalent to the pH at which 50% drug is ionized and 50% is unionized For a weak acid if pH –pKa is low it will be more nonionized and better absorbed For a weak base if pH –pKa value is high it will be more nonionized and better absorbed