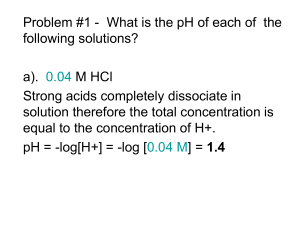
For acids
advertisement

Dr. Bilal Al-jaidi Medicinal Chemistry Is the science that deals with the design and development of pharmaceutical agents that has a desired biological effect on human body and other living systems. Drug Is a compound that interact with a biological target to produce a biological response: Biological system: Human, bacteria, fungi,… Biological response: desired or undesired. Sugar, salt, pesticides, herbicides, can be considered as drugs. Food and fizzy drinks also considered as drugs. Others define drugs as molecules used as medicine or as a components in medicines to diagnose, cure or prevent disease. Medicinal chemistry also involves isolation of compounds from natural sources or the synthesis of new molecules the trying to investigate the relationships between the chemical structure of these compounds and their biological activities. The Ideal drug is: Not toxic. Effective and potent. Selective. Easily administered. Cheap In Reality, There is no Ideal drug. Penicillin: one of the safest and most active antibiotics……BUT….. Resistance developed to most of them. Morphine: very effective pain killer….. BUT…. May cause tolerance, addiction and respiratory depression. Heroin: the best pain killer we know….BUT…. addiction developed (still used in terminal cancer). Drug might be harmful at higher doses: Therapeutic index: it is the ratio of the dose leads to toxic effect in 50% of cases to that leads to therapeutic effect in 50% of the cases. Large therapeutic index…… safer drug. narrow therapeutic index…… more toxic drug. Poisons can be drugs at lower doses: Arsenicals: very toxic but used as antiprotozoal agents. Tubocurarine: used as muscle relaxant. Selective Toxicity Selective Drugs are those that show toxicity against abnormal cells without affecting normal cells. Degrees of selectivity: No effect on normal host cells. Killing certain microbial strain without affecting others. Targeting certain metabolic pathway with affecting others Drug Targets they are macromolecules (receptors, enzymes, DNA or transport proteins). Drugs interact and bind to the binding sites through intermolecular bonds (ionic, H-bonds, Van Der Waals, dipole-dipole and hydrophobic). The bonds mainly are weak bonds, therefore this binding is reversible in most of the cases. In medicinal chemistry: Pharmacokinetic: How the drug distribute and reach its target (ADME) and what will happen to the drug Pharmacodynamic: How the drug interact with its target. Pharmacodynamics – what the drug does to the body: – What is the therapeutic effect of the drug? – How does it exert its effect? – How does the drug interact with the target? – Can the effect be modified? Pharmacokinetics – what the body does to the drug: How do you get it into the body? How long does it take to exert its action? How long does it stay in the body? Where does it go to in the body? Is it metabolised to another form? How do we analyze and detect it? The [plasma]-time curve after drug administration Pharmacokinetics Which route? Which formulation? Drug administered Drug absorbed Metabolic inactivation Which barriers to cross? Gut, skin, lungs? Stability at the site of absorption? Pool of non-available Drug in the tissues Plasma-protein binding? • Electrostatic charge Tissue-protein binding? Fat sequestration? • Lipophilicity [Volume of distribution] available Drug in the plasma Passive diffusion? Active transport? Blood-brain barrier penetration? Drug at the site of action Excretion What do we mean by: Oral availability Oral stability Tissue availability Orally active Oral availability Oral availability or bioavailability measures the fraction of the drug being absorbed into the blood circulation. Factors affecting oral availability: Chemical nature of drug (lipophilicity and ionization state). Water solubility. Oral stability. Physiological factors. Oral stability Oral stable drugs must be: Chemically stable toward the GIT conditions; acidic stomach and basic intestine. Enzymatically stable: stable toward the digestive and metabolizing enzymes such as esterase, amidase and oxidase enzymes. If the drug is orally unstable it will not be available to be absorbed…..low oral availability. Orally activity Orally active agents are drugs either active locally in the GIT lumen (such as in the case of gastroenteritis) or must be absorbed into the blood circulation. Factors affecting oral activity: Chemical and enzymatic stability of drugs. The physiological nature of the GIT lumen. The same factors affecting the oral availability in the case of systemically active agents. Tissue Availability Tissue availability means the amount of the drug that reached the site of action or the target tissue. In most of the cases, tissue availability is lower than the oral availability due to one of the following factors: Extensive drug metabolism. Blood protein binding. Rapid drug excretion. Fat deposition of drug. The many barriers the drug have to penetrate to reach the site of action. The molecular properties of drugs It is the physicochemical properties of drugs. These properties fundamentally affect every thing the drug does to the body (the pharmacodynamic aspects) and what the body does to drugs (the pharmacokinetic aspects). the molecular properties also determine which dosage form and the route of administration is suitable for the given drug. Molecular properties of interests 1. 2. 3. 4. 5. Partition coefficient. Dissociation constant (degree of ionization). Solubility (aqueous and fat solubility). Chemical stability. Biological stability (metabolic profile of drugs). Physicochemical properties of drugs • Partition coefficient • Lipophilicity/hydrophilicity • Ionisation/dissociation constant • Strong or weak acids/bases • Salt formation • Solubility • Water-soluble salts • Lipid soluble esters • • • Stability Chemical degradation – oxidation, hydrolysis, light Enzyme degradation (metabolism) esterases, amidases, cytochrome P450 Pharmacokinetic properties Drug administration: How is the drug to be formulated? If as an injection, is it soluble in aqueous solution? If as a tablet, will it dissolve when released in the gut? Drug absorption: can the drug pass through the barrier membranes in the GIT? Can it pass through the skin barriers? These barriers are made up in a large part by lipids, so the drug must be sufficiently fatsoluble/ unionized to diffuse through them. Membranes have phospholipids bilayer that act as barriers to the movement of drugs within the body Pharmacokinetic properties Drug metabolism: metabolism increases the water solubility of drugs by enzymatically introducing polar functional groups so that they can be excreted: what is the chemistry of the drug? How fast is it inactivated? Is it converted into more active or even toxic components? Drug excretion: the kidney excretes water-soluble metabolites. Pharmacodynamic properties It is what the drug does to the body. How does the drug interact with biological receptors or enzymes and what are the factors affecting this binding. Lipophilicity/hydrophilicity of drugs Acceptor H O H CH3 H O Donor O H Acceptor H O Donor Partition coefficient Is the measurement of the drug water solubility. Partitioning means that the drug will be divided in parts between water and oil layer. P = [Co ]/[Cw] LogP = Log[Co ]/[Cw]. LogP > 2 lipophilic drug. LogP < 2 hydrophilic drug LogP only applied for neutral compound Low logP….. Low penetration to CNS High logP….. Low water solubility…. Not suitable for oral administration Ionisation and dissociation • • ACIDS ARE PROTON DONORS acid is a substance that can dissociate to produce H+ and a negative ion (anion) which is called a conjugate base i.e.: • • BASES ARE PROTON ACCEPTORS Bases can accept a proton to form the positively charged cation ( conjugate acid of the base). pH in different body compartments Plasma Buccal cavity Stomach Duodenum Jejunum & ileum Colon 7.35 – 7.45 6.2 – 7.2 1.0 – 3.0 4.8 – 8.2 7.5 – 8.0 7.0 – 7.5 O O Ka H3C H3C H OH O [CH 3COO ][H ] Ka [CH 3COOH ] Ka for CH3CO2H is approximately 10-5 Ka 1 10 5 i.e. only 1 molecule in 100,000 is DISSOCIATED (ionised). -log10Ka = pKa So pKa for acetic acid is 5 O O Ka HO S OH HO O S O H O [ HSO4 ][H ] Ka [ H 2 SO4 ] Ka for H2SO4 is approximately 105 105 Ka 1 i.e. 100,000 molecules are DISSOCIATED (ionised) for every one undissociated. The pKa of H2SO4 is therefore -5 [ PhCH2 NH 2 ][H ] Ka [ PhCH2 NH 3 ] Ka for PhCH2NH3+ is approximately 10-9 (pKa = 9) Ka i.e. only 1 molecule in 1,000,000,000 is DISSOCIATED (UNIONISED). A weak conjugate acid does not willingly donate its proton (1 molecule in 1,000,000,000 donates a proton) Therefore a strong base willingly accepts a proton (1,000,000,000 molecules accept a proton for every one) 1 10 9 pKa is a different term than pH pH is simply a measure of the [H+] concentration in a given solution pH = 1 ...........the environment is acidic pKa = 1 DOES NOT mean an acidic molecule pH = 14 ............the environment is basic pKa = 1 DOES NOT mean a basic molecule You can’t tell from the pKa value whether the species in question is acidic or basic Weak a cid Strong base Cl Cl Cl C Cl OH COOH Cl C pKa = 0.9 COO H Cl O H Which one is the stronger acid? pKa = 10.0 Considering Ka values relates ratio of products to reactants Cl Cl C Cl OH pKa = 0.9 Cl COOH Cl C COO Ka = 10-0.9 H [Cl3COO ][H ] Ka [Cl3COOH ] 1 K a 0 .9 10 Cl O pKa = 10.0 H [ PhO ][H ] Ka [ PhOH] Ka = 10-10 1 K a 10 10 Phenols are weaker acids than acetates NH2 NH3 NH H NH2 pKa = 0.5 pKa = 9.0 H Which one is the stronger base? pKa = 0.5 Ka = 10-0.5 NH2 NH3 NH [ Ph2 NH ][H ] Ka [ Ph2 NH 2 ] 1 K a 0 .5 10 H NH2 pKa = 9.0 H Ka = 10-9 [ PhCH2 NH 2 ][H ] Ka [ PhCH2 NH 3 ] Ka 1 10 9 Aromatic amines are weaker bases than aliphatic amines • We can quantify how pH changes the ratio of dissociated to undissociated species as follows: pH pKa log10 1 0p Hp K a Dissociated Undissociated Dissociated Undissociated antilog pH pKa Dissociated Undissociated What is the importance of studying the pKa values for Acidic and basic drugs? • only the unionised form of a drug can partition across biological membranes (providing the unionized form is lipophilic) • the ionised form tends to be more water soluble [required for drug administration and distribution in plasma] PARTITIONING OF ACIDS AND BASE Consider drugs that are acids, for example RCOOH, which has a pKa of 4.0 Biological Membrane Gut Contents RCOOH H • • • + RCOO RCOOH X Drug Absorption No Drug Absorption If the pH shifts the balance towards the unionized/undissociated form, the drug would be absorbed. If the pH shifts the balance towards the ionized/dissociated form, the drug would not be absorbed. Assume the pH of the stomach is 2.0 and the pH of the small intestine is 8.0. Where would you expect absorption to take place from? PARTITIONING OF ACIDS AND BASE Biological Membrane Gut Contents RCOOH H + RCOO RCOOH X + RNH2 RNH3 No Drug Absorption Biological Membrane Gut Contents H Drug Absorption RNH2 X No Drug Absorption Drug Absorption So we should expect that this compound will not be readily absorbed though the lipophilic membranes although it is in the unionized form Remember the followings For acids: 1. a high pka means the species is predominantly unionised, is a bad proton donor, and a weak acid 2. a low pka means the species is predominantly ionised, is a good proton donor, and a strong acid 3. pH < pKa by 2 units, 99% unionised . 4. pH > pKa by 2 units, 99% ionised For bases: 1. a high pka means the species is predominantly ionised, is a good proton acceptor, and a strong base 2. a low pka means the species is predominantly unionised, is a bad proton acceptor, and a weak base 3. pH < pKa by 2 units, 99% ionised 4. pH > pKa by 2 units, 99% unionised Common acidic functional groups in pharmaceutical chemistry and their pKa values O R 4-5 OH 10 OH 9.9 <2 O R NH 8-10 R O Common basic functional groups in pharmaceutical chemistry and their pKa values R NH2 10.0 NH2 4.6 R NH R 10.6-11.0 HN N 6.5 R N R R 9.8-10.8 N 5.2 Common neutral functional groups in pharmaceutical chemistry R R O NH2 R R O O R O NH O R O R HN N R R R OH N H R Molecular properties and routes of administration • • • • • • • Oral rectal vaginal topical parenteral Respiratory The molecular properties of the drug must be determined before any route can be considered, but other factors are important Factors to consider when choosing a route of administration • • • • • • • molecular properties of the drug physiological nature of the route patient compliance onset of action the condition being treated systemic or local effect (side effects) metabolism Oral administration and absorption • If a drug is to be absorbed through the mucosal membranes that line the gut, then it must be in its lipophilic unionised form to partition out of the aqueous medium. • acidic drugs tend to be absorbed more rapidly from the stomach, whilst basic drugs must pass through to the small intestine before absorption. • The partition co-efficient of the unionised form will also determine how much is absorbed. • The absorption phase of the dose-response curve is therefore heavily influenced by the pKa and log P of a drug. Oral administration and absorption Orally administered drugs must have: • • • • logP < 5. No more than 10 hydrogen bond acceptors. No more than 5 hydrogen bond donors. A molecular weight less than 500 Dalton. These points are called ‘’ Lipinski’s rule of five’’ • Not more than 7 rotatable bonds. Practice question Loratadine is an orally available drug, it has a pKa of 5, answer the followings according to its structure: Is it basic, acidic or neutral compound? Calculate the % ionization: In stomach (pH = 2): In intestine (pH = 8): Based on your calculation, from where do you think loratadine will be absorbed?