Explain the Strength of Organic Acids & Bases
advertisement

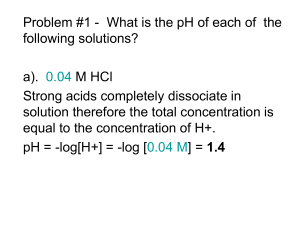
p.01 Acid-Base Eqm (3): Explain Explain the the Strength Strength of of Organic Organic Acids & Bases Review Questions Q.1: pKb of CH3NH2 (an organic weak base) at 298K is 4.75. Find the pH of 0.05M CH3NH2 at 298K. Kb = x2 0.05 – x x2 = 10-4.75 = 1.77810-5 x = 9.4310-4 0.05 pOH = -log(9.43 10-4) = 3.03 pH = 14 – pOH = 11.0 C. Y. Yeung (CHW, 2009) Q.2: p.02 25.0 cm3 of a monobasic organic weak base B(aq) requires 20.25 cm3 of 0.50M HCl(aq) for complete neutralization. Given that the pH of the B(aq) at 298K is 11.25, what is Kb of B at 298K? (20.25/1000)0.50 = 0.405 M conc. of B(aq) = (25.0/1000) B + H 2O HB+ + OH- at start 0.405 0 0 at eqm 0.405 – x x x As pH = 11.25, i.e. pOH = 14 – 11.25 = 2.75 x = [OH-] = 10-2.75 = 1.77810-3 Kb = (1.77810-3)2 = 7.8410-6 M 0.405 – 1.77810-3 p.03 Stability of Conjugate Base … e.g. CCl3COO- is the conjugate base of CCl3COOH i.e. CCl3COO- is stable, thus it is less likely to react with H3O+ to regenerate CCl3COOH. CCl3COO- is stabilized by “negative inductive effect”. CCl3COO- + H3O+ CCl3COOH + H2O Cl electronegative atom !! Cl C Cl O C - O The -ve charge is dispersed /delocalized over the anion. The -ve charge is less concentrated on the O atom. p.04 Conjugate Base may be stabilized by “resonance effect” OH O + + H2 O H3 O + phenoxide phenol O- - O O - O - (resonance structures) The -ve charge is delocalized over the aromatic ring. (resonance effect) i.e. phenoxide ion is stabilized by resonance effect. pKa of Phenol = 9.95 pKa of Ethanoic acid = 4.76 How to explain …? p.05 stronger acid! Although CH3COO- is destabilized by positive inductive effect, it is stabilized by resonance effect! - O O CH3 C O CH3 C O both are electronegative! i.e. –ve charge is shared equally. In the case of phenoxide, delocalization of –ve charge by resonance is less effective, because the O atom is more electronegative than the carbon atoms in aromatic ring. The –ve charge is more delocalized in CH3COO-, and thus it is more stable. CH3COOH is the stronger acid. p.06 Strength of Base … (depends on the availability of lone pair e-.) availability of lone pair e- is increased by +I effect push e- CH3 N H stronger base (than NH3)! availability of lone pair e- is decreased by resonance. H Methylamine (pKb = 3.38) N phenylamine / aniline (pKb = 9.40) + N H N H - H H weaker base (than NH3)! H Ref: pKb of NH3 = 4.80 - H - + N H H + N H (resonance structures) H Advanced Understanding on the Strength of Organic Acid (1) --- 4-nitrophenol (p-nitrophenol) How can 4-nitrophenoxide ion be stabilized by resonance effect? O O O O N+ O O N+ O O O O - O O - Therefore, it is stronger than phenol. N+ O + N O O The -ve charge on the p-nitro phenoxide ion is more dispersed than that on the phenoxide ion. N+ N+ O O O pKa of phenol = 9.95 pKa of p-nitrophenol = 7.14 a.1 Advanced Understanding on the Strength of Organic Acid (2) --- 3-nitrophenol (m-nitrophenol) a.2 Is the pKa of m-nitrophenol smaller than p-nitrophenol? O O The -ve charge cannot be dispersed over the nitro group. + N O O + N O O O O (But it is still stronger than phenol due to negative inductive effect.) - + N O O Therefore, it is weaker than p-nitrophenol, and its pKa is larger. + N O O pKa of phenol = 9.95 pKa of p-nitrophenol = 7.15 pKa of m-nitrophenol = 8.35 Advanced Understanding on the Strength of Organic Acid (3) --- p-hydroxybenzaldehyde a.3 How can the conjugate base be stabilized by resonance effect? O O O O - C C H O H O C O O - O C H O C O H The -ve charge is dispersed over the anion, p-hydroxy benzaldehyde is acidic. Therefore, it is stronger than phenol. O C H - H pKa of phenol = 9.95 pKa of p-hydroxybenzaldhyde = 7.66 a.4 Look at their pKa values again …… OH OH + N O O N+ O O pKa (at 298K) 9.95 7.15 OH OH 8.35 C H O 7.66 nitro group (– NO2) at the p-position stabilizes the conjugate base by delocalizing the – ve charge through: negative inductive effect resonance effect a.5 Compare the pKb values of m-nitroaniline, p-nitroaniline and aniline. NH2 NH2 NH2 + N O O N+ O O pKb (at 298K) 9.40 12.9 11.6 The availability of lone pair e- on the –NH2 group is reduced by : negative inductive effect resonance effect p.07 In Conclusion …. Stronger Acid: conjugate base stabilized by - I effect / resonance effect. (delocalization of –ve charge) Weaker Acid: conjugate base destabilized by + I effect. (localization of –ve charge) Stronger Base: availability of lone pair e- increased by + I effect. (localization of –ve charge) Weaker Base: availability of lone pair e- decreased by - I effect / resonance. (delocalization of –ve charge) p.08 More practice on the calculation of Ka, Kb and pH Q.1: At body temperature, human blood pH is 7.40. Lactic acid, CH3CH(OH)COOH, is produced in muscle tissues during physical exertion. Whether the lactic acid produced exists mainly in form of “undissociated molecule” or “dissociated lactate ions”? (Given: Ka of lactic acid at body temperature = 8.5010-4 mol dm-3) Ka = [H3O+][CH3CH(OH)COO-] [CH3CH(OH)COOH] pKa = pH – log 2.14 104 = [CH3CH(OH)COO-] [CH3CH(OH)COOH] [CH3CH(OH)COO-] With a given Ka, the ratio of [salt] and [acid] could be found from the pH value. [CH3CH(OH)COOH] Lactic acid formed is mainly in form of dissociated lactic ions. Q.2: Calculate the pH value of the resultant solution for 10cm3 of 0.20M CH3COOH are mixed with 10cm3 of 0.40M CH3COONa. (Ka = 1.7510-5 mol dm-3) After mixing, 1.7510-5 new [CH3COOH] = 0.10M new [CH3COONa] = 0.20M x(0.20+x) = 0.10 – x x = 8.75 10-6 pH = 5.06 An acid-salt mixture (acid and salt have comparable conc.) ACIDIC BUFFER! p.09 Q.3: Calculate the pH value of the resultant solution for 10cm3 of 1.00M NH3 are mixed with 10cm3 of 1.00M NH4Cl. (Kb = 1.7810-5 mol dm-3) After mixing, 1.7810-5 new [NH3] = 0.50M new [NH4+] = 0.50M x(0.50+x) = 0.50 – x x = 1.78 10-5 pOH = 4.75 pH = 9.25 A base-salt mixture (base and salt have comparable conc.) BASIC BUFFER! p.10 p.11 Assignment Lab report [due date: 6/4(Mon)] Book 3A p.141 Q.12, 13, 14 [due date: 20/4 (Mon)] Pre-Lab: Expt. 13 Analysis of Two Commercial Brands of Bleaching Solution Next …. Acidic Buffers and Basic Buffers (Book 2 p. 153 – 163)