Chapter 20: Electrochemistry
advertisement
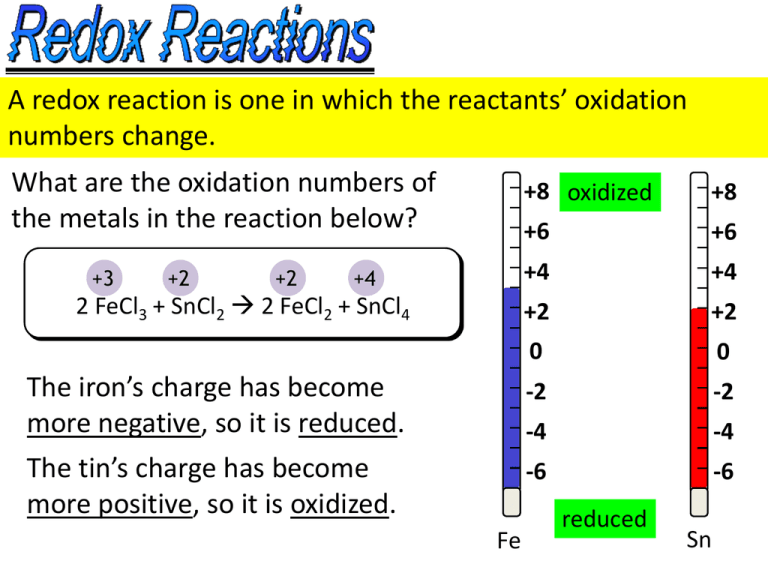
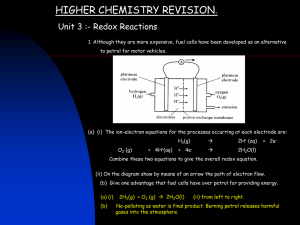
A redox reaction is one in which the reactants’ oxidation numbers change. What are the oxidation numbers of the metals in the reaction below? +8 oxidized +8 +6 +6 +4 +4 +2 +2 0 0 The iron’s charge has become more negative, so it is reduced. -2 -2 -4 -4 The tin’s charge has become more positive, so it is oxidized. -6 -6 +3 +2 +2 +4 2 FeCl3 + SnCl2 2 FeCl2 + SnCl4 Fe reduced Sn Which reactant is oxidized and which is reduced in the following? 0 +2 -1 +4 -1 1) Cl2 + SnCl2 SnCl4 Synthesis oxidized reduced 0 +1 +2 0 2) Cu + 2AgNO3 Cu(NO3)2 + 2Ag Single-replacement oxidized Note that a redox reduced reaction can also be +5 another type of -2 -1 0 3) 2KClO3 2KCl + 3O2 Decomposition reaction. oxidized reduced KClO3 +1 + x + 3(–2) = 0 x = +5 Balancing Redox Reactions +7 Rxn: +3 MnO4 + C2O4 – 2– Mn2+ +4 + CO2 (acidic conditions) 1. Determine the oxidation number of the redox active species. 2. Split the redox reaction into two half reactions (remember OIL RIG) and add in the #e– being transferred. Red: 2 8 H+ + 5e– + MnO4– Mn2+ + 4 H2O Ox: + 5 C2O42– 2 CO2 + 2e– 16 H+ + 2 MnO4– + 5 C2O42– 2 Mn2+ + 10 CO2 + 8 H2O 3. Balance the oxygens with H2O and the hydrogens with H+. 4. Multiply both half-reactions by a factor that will make the #e– transferred equal. 5. Add the two half-reactions to get the final balanced redox rxn. Balancing Redox Reactions Under Basic Conditions 1. Balance reaction using acidic conditions. 16 H+ + 2 MnO4– + 5 C2O42– 2 Mn2+ + 10 CO2 + 8 H2O +16 OH– +16 OH– 8 16 H2O 2. Add OH– to both sides to neutralize the H+. 3. Cancel out excess water and rewrite reaction equation. 8 H2O + 2 MnO4– + 5 C2O42– 2 Mn2+ + 10 CO2 + 16 OH– Voltaic (alt. Galvanic) Electrochemical Cells ZnZn2+Cu2+Cu AnodeCathode 0.92 Ecell = EA + EC Ecell = 0.763V + 0.153 V 0.916 V Salt bridge EA = +0.763 V Oxidation takes place at the anode. EC = +0.153 V Reduction takes place at the cathode. Example: What are the standard cell potentials for the folllowing? Al3+ + 3e– Al Eo= –1.66 V Br2 + 2e– 2Br– Ag+ + e– Ag Eo= +0.80 V Eo= +1.06 V 1. AlAl3+Ag+Ag Ox: Al Al3+ + 3e– Red: Ag+ + e– Ag +1.66 V +0.80 V +2.46 V 2. AgAg+Br2Br– Ox: Ag Ag+ + e– –0.80 V Red: Br2 + e– 2 Br– +1.06 V +0.26 V Note: Voltaic cells ALWAYS have positive cell emf’s (voltages). Some terminology emf– Electromotive force; force causing e– to move SHE– Standard hydrogen electrode; Eo = 0 V by definition. Standard conditions– Solution concentrations = 1 M and gas pressures = 1 atm Oxidizing agent– is reduced during redox rxn Reducing agent– is oxidized during redox rxn Faraday’s constant = 96485 C/mol e– transferred R = 8.314 J/mol·K 1 A = 1 C/s 1 V = 1 J/C (C Coulomb = a quantity of charge) Table of Standard Reduction Potentials Good oxidizing agent = large, positive reduction potential SHE Good reducing agent = large, negative reduction potential A voltaic cell generates current as a result of a spontaneous redox reaction. The equations relating E, DG and K are: DG = -nFE K e DG RT e nFE RT lnK = -DG/RT = nFE/RT R = 8.314 J/mol·K, T = temperature in Kelvin, F = 96485 C/mol n = # electrons transferred in reaction 20.58 What are DG and K for: 2VO2+ + 4H+ + 2Ag VO2+ + 2H2O + 2Ag+ Ecell = EC + EA = 1.00 V + (–0.799 V) = 0.201 V n = 2 e– (Remember 1 V = 1 J/C) DG = -nFE = -(2 e–)(96485 C/mol e–)(0.201 V) = -38787 J/mol K = e–DG/RT = e(38787 J/mol)/(8.314 Jmol*298K) = e15.7 = 6294875 Operating under non-standard conditions: 0.0592 [products] o log Nernst Eq. = Ecell Ecell [reactants] n 20.50 What is the cell potential for the following if [Ce4+]=2.0M, [Ce3+] = 0.010 M and [Cr3+] = 0.010 M? 3Ce4+ + Cr(s) 3Ce3+ + Cr3+ RED n = 3e– OX Eo = EC + EA = +1.61 V + +0.74 V = 2.35 V E = 2.35 V – (0.0592/3)log [0.010 M]3[0.010 M] [2.0 M]3 = 2.53 V Because [reactants]>>[products] AND the reaction has a large positive Eo, the emf has increased to produce more product. Electrolysis: Decomposition of a compound by passing electricity through it. 20.80 Metallic magnesium can be made by the electrolysis of molten MgCl2. What mass of Mg is formed by passing a current of 5.25 A through molten MgCl2 for 2.50 days? 24 hrs 60 min 60 s • Calculate total time:2.50days x x x 216000s day hr min • Calculate total charge passed = 5.25 C/s * 216000 s = 1134000 C • Divide by 2 because it takes 2e–/equiv Mg2+= 567000 C • Calculate mol Mg = 567000 C /(96485 C/mol) = 5.877 mol Mg(s) • Calculate grams Mg = 5.877 mol Mg(s) * 24.305 g/mol = 142.8 g Ans: 143 g of Mg will be formed