Spontaneity of Redox Reactions
advertisement
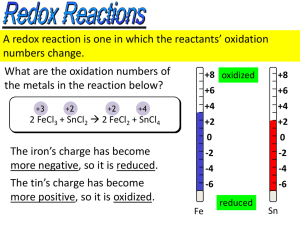
Chapter 20 Overview • Oxidation-Reduction reactions » Balancing Redox Reactions • Half-Reaction method • Acidic Solution • Basic Solution • Voltaic Cells • Cell EMF--standard reduction potentials • Oxidizing & Reducing reagents • Spontaneity of Redox reactions • Effect of Concentration » Nernst Equation » Equilibrium Constants • Commercial Voltaic Cells • Electrolysis » Quantitative Aspects » Electrical Work Redox Reactions • Involve a transfer of electrons » Oxidation loss of one or more electron(s) • oxidation state will increase » Reduction gain of one or more electron(s) • oxidation state will decrease » Must occur simultaneously Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Zn Zn2+(aq) + 2e- Cu2+(aq) + 2e- Cu(s) oxidation ½ rxn reduction ½ rxn You must know oxidation states: (Review: Section 8.10) • What are the oxidation states of each atom in the following: » » » » H2 CO ClO2HC2H3O2 H 0 C +2, O -2 Cl +3, O -2 H +1, C1 +3, C2 -3, O Balancing Redox Reactions • Mass balance must be observed • e--transfer must be balanced • Simple reactions: » Sn2+ + Fe3+ Sn4+ + Fe2+ • Sn2+ Sn4+ + 2e• Fe3+ + e- Fe2+ x 2 2Fe3+ + 2e- 2Fe2+ oxidation ½ rxn reduction ½ rxn Sn2+ + 2Fe3+ Sn4+ + 2Fe2+ • Reactions involving H & O in acid: » MnO4- + C2O42- Mn2+ + CO2 » write both ½ reactions • MnO4• C2O42- Mn2+ CO2 » mass balance (all except H & O) • MnO4• C2O42- Mn2+ 2CO2 » add H2O & H+ to balance O & H • 8H+ + MnO4 • C2O42- 2CO2 Mn2+ + 4H2O » balance charge by adding electrons • 5e- + 8H+ + MnO4 • C2O42- 2CO2 + 2e- Mn2+ + 4H2O » balance electrons transferred • 10e- + 16H+ + 2MnO4 • 5C2O42- 10CO2 + 10e- 2Mn2+ + 8H2O » add half reactions • 16H+ + 2MnO4-+ 5C2O42- 10CO2 + 2Mn2+ + 8H2O »check the balance • Reactions in base: MnO4- + CN- CNO- + MnO2 » use exactly the same process • H2O + CN- CNO- + 2H+ + 2e• 3e- + 4H+ + MnO4- MnO2 + 2H2O » since H+ cannot exist in basic solution, add OH• 2OH- + CN- CNO- + H2O + 2e• 3e- + 2H2O + MnO4- MnO2 + 4OH- » balance electrons transferred & sum • 6OH- + 3CN- 3CNO- + 3H2O + 6e• 6e- + 4H2O + 2MnO4- 2MnO2 + 8OH- » 3CN- + H2O + 2MnO4- 2MnO2 + 3CNO- +2OH- »check balance Voltaic Cells • A spontaneous redox reaction that does work • Anode » electrode at which oxidation occurs » loses mass » electrons released, sign is negative • Cathode » electrode at which reduction occurs » gains mass » electrons consumed, sign is positive Cell EMF • Difference in potential energy of electrons at the anode and cathode » Diff. in potential energy per electrical charge measured in volts »1V = 1 J C • Potential difference = EMF, electromotive force • Ecell = cell potential = cell voltage » Eºcell = cell potential under std. conditions • 1 M, 1 atm, 25 ºC • Standard reduction potentials » E ºred in tables » E ºcell = E ºred (cathode) - E ºred (anode) • Based on “standard hydrogen electrode” » 2H+(aq, 1M) + 2e- H2(g, 1atm) E ºred = 0 V » Zn(s) + 2H+(aq) Zn2+(aq) + H2(g) E ºcell = 0.76 V » 0.76 V = 0 V - E ºred (anode) • Zn2+(aq, 1M) + 2e- Zn(s) E ºred (anode) = -0.76 V Problem: • Calculate Eºcell for • 2Al(s) + 3I2(s) 2Al3+(aq) + 6I-(aq) » Anode: » Cathode: 2Al 2Al3+ + 6e- 3I2 + 6e- 6I- • Eºcell = E ºred (cathode) - E ºred (anode) • E ºcell = 0.54 V - (-1.66 V) • E ºcell = 2.20 V • Note: stoichiometric coefficient does not affect the value of the E ºred (it is an intensive property) • E ºox = - E ºred • 2Al(s) + 3I2(s) 2Al3+(aq) + 6I-(aq) • 2Al 2Al3+ + 6e- E ºox = +1.66 V • 3I2 + 6e- 6I- E ºred = +0.54 V • E ºcell = E ºox + E ºred = 2.20V • The more positive the E ºcell the more driving force for the reaction Oxidizing/Reducing Agents • Oxidizing agents cause oxidation » oxidizing agents are reduced » the more (+) the E ºred the better the ox. agent • Reducing agents cause reduction » reducing agents are oxidized » the more (-) the E ºred the better the red. agent • Which is the better oxidizing agent? • NO3- + 4H+ + 3e- NO + 2H2O E ºred 0.96 V • Ag+ + e- Ag E ºred 0.80 V • Cr2O72- + 14H+ + 6e- 2Cr3+ + H2O E ºred 1.33 V • Which is the strongest reducing agent? • I2 + 2e- 2I- Eºred +0.54 V • Fe2+ + 2e- Eºred -0.44 V Fe • MnO4- + 8H+ + 5e- Mn2+ + 4H2O Eºred +1.51 V Spontaneity of Redox Reactions • Spontaneous redox rxns have positive potentials • Non-spontaneous redox rxns have negative potentials • Is this rxn spont. or non-spont.? » MnO4- + 8H+ + 5Fe2+ 5Fe3+ + Mn2+ + 4H2O • Fe2+ Fe3+ + 1e• MnO4- + 8H+ + 5e- Mn2+ + 4H2O • E ºox + E ºred = + 0.74 v Yes Eºox = -0.77 v E ºred = +1.51 v EMF & Free Energy • If both DG & E are a measure of spontaneity, they must be related » DG = - nFE • F is Faraday’s constant 1 F = 96,500 J/v mol e- • remember: 1 C = 1 J/v • n = mol e- transferred » In the standard state DGº = - nFEº • Calculate the standard free energy change for Hg + 2Fe3+ Hg2+ + 2Fe2+ » n = 2 mol electrons transferred • Hg Hg2+ + 2e- Eox = - 0.854 v • 2Fe3+ +2e- 2Fe2+ Ered= + 0.771 v • Ecell = - 0.083 v » DG = - (2 mol e-)(-0.083 v)(96,500 J/v mol e-) » = + 16 kJ Concentration & Cell EMF • Nernst Equation » relationship between DG & concentrations • DG = DGº + RT ln Q Q = [prod]x/[react]y » substitute -nFE for DG • E = Eº - (RT/nF) ln Q or • E = Eº - (2.303 RT/nF) log Q • 2.303 RT/F = 0.0592 v-mol e- at std. temp. • E = Eº - (0.0592/n) log Q • Calculate the emf that the following cell generates when [Mn2+] = 0.10 M & [Al3+] = 1.5 M 2Al + 3Mn2+ 2Al3+ + 3Mn • Eº = + 0.48 v • E = (+ 0.48 v) - (0.0592 v/ 6) log [(1.5)2/(0.10)3] • E = + 0.45 v • when [Mn2+] = 1.5 M & [Al3+] = 0.10 M • E = (+ 0.48 v) - (0.0592 v/ 6) log [(0.10)2/(1.5)3] • E = + 0.51 v Equilibrium Constants • Remember DG = DGº + RT ln Q, if Q = K, then DG = 0, therefore -nFE = 0 and • 0 = Eº - (RT/nF) ln K or • 0 = Eº - (0.0592/n) log K • K can be calculated from cell potentials • log K = nE º/0.0592 • Calculate the equilibrium constant, K, for 2IO3- + 5Cu + 12H+ I2 + 5Cu2+ + 6H2O • Eº = + 0.858 v • n = 10 mol e- transferred • log K = nEº/0.0592 • log K = 145 • K = 1 x 10145 Voltaic Cells • Lead storage battery » PbO2 + SO4-2 + 4H+ + 2e- PbSO4 + H2O Pb + SO42- PbSO4 + 2e• Ecell = + 2.041 v • Dry cell » NH4+ + 2MnO2 + 2e- Mn2O3 + 2NH3 + H2O Zn Zn2+ + 2e• In an alkaline cell the NH4Cl is replaced with KOH • Ni-Cd » NiO2 + 2H2O + 2e- Ni(OH)2 + 2OH- Cd + 2OH- Cd(OH)2 + 2e- • Fuel cells » 4e- + O2 + 2H2O 4OH2H2 + 4OH- 4H2O Electrolytic Cells • Redox reactions that are not spontaneous • Must be driven by an outside source of electrical energy • Cathode » reduction occurs » by sign convention, is negative • Anode » oxidation occurs » by sign convention, is positive Quantitative Aspects • Redox reactions occur in stoichiometric relationship to the transfer of electrons • Electrons put into a system through electrical energy, can be quantized » Coulomb = quantity of charge passing through electrical circuit in 1 s at 1 ampere (A) current • Coulomb = (amp) (seconds) Problem: Calculate the mass of Mg formed upon passage of a current of 60.0 A for a period of 4.00 x 10 3 s. • MgCl2 Mg + Cl2 » Mg2+ + 2e- Mg 2Cl- Cl2 + 2e- • we are concerned with the reduction • (60.0 A)(4 x 103s)(1C/1 A-s) = 2.4 x 105 C • (2.4 x 105 C)(1 mol e-/ 96,500 C) = 2.49 mol e- • (2.49 mol e-)(1 mol Mg/2 mol e-) = 1.24 mol Mg • (1.24 mol Mg)(24.3 g/mol) = 30.1 Mg Electrical Work • DG = wmax DG = - nFE wmax = - nFE • Max work proportional to potential • = wmax •J = - n F E (mol) (C/mol) (J/C) • Electrical work = (watt) (time) • 1 watt (W) = 1 J/s • 1 kWh = 3.6 x 106 J or watt-s = J










![Redox_equations[1].](http://s2.studylib.net/store/data/005611618_1-b55ee2e3a52621e48ab97cc179174553-300x300.png)