Unit 3 Redox Reactions
advertisement
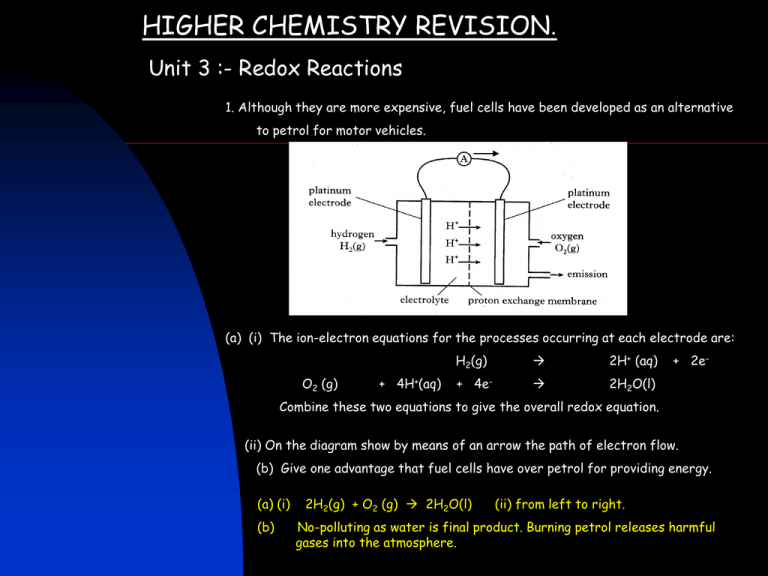
HIGHER CHEMISTRY REVISION. Unit 3 :- Redox Reactions 1. Although they are more expensive, fuel cells have been developed as an alternative to petrol for motor vehicles. (a) (i) The ion-electron equations for the processes occurring at each electrode are: O2 (g) + 4H+(aq) H2(g) 2H+ (aq) + 4e- 2H2O(l) + 2e- Combine these two equations to give the overall redox equation. (ii) On the diagram show by means of an arrow the path of electron flow. (b) Give one advantage that fuel cells have over petrol for providing energy. (a) (i) (b) 2H2(g) + O2 (g) 2H2O(l) (ii) from left to right. No-polluting as water is final product. Burning petrol releases harmful gases into the atmosphere. 2. Sodium sulphite is a salt of sulphurous acid, a weak acid. Identify the two statements which can be applied to sodium sulphite You may wish to refer to the data booklet. A It can be prepared by a precipitation reaction. B It can be prepared by the reaction of sulphurous acid with sodium carbonate. C In solution, the pH is lower than a solution of sodium sulphate. D In redox reactions in solution, the sulphite ion acts as a reducing agent. E In redox reactions in solution, the sodium ions are oxidised. Statements in boxes A and D 3. (a) Some carbon monoxide detectors contain crystals of hydrated palladium(II) chloride. These form palladium in a redox reaction if exposed to carbon monoxide. CO(g) + PdCl2.2H2O(s) CO2 (g) + Pd(s) + 2HCl(g) + H2O(l) Write the ion-electron equation for the reduction step in this reaction. (b) Another type of detector uses an electrochemical method to detect carbon monoxide. At the positive electrode. CO(g) + H2O(l) CO2 (g) + 2H+(aq) + 2e– At the negative electrode O2 (g) + 4H+ (aq) + 4e – 2H2O(l) Combine the two ion-electron equations to give the overall redox equation. (a) Pd2+ + 2e- Pd (b) 2CO + O2 2 CO2 4. The glucose content of a soft drink can be estimated by titration against a standardised solution of Benedict’s solution. The copper(II) ions in Benedict’s solution react with glucose as shown. C6H12O6 (aq) + 2Cu2+(aq) + 2H2O(l) Cu2O(s) + 4H+(aq) + C6H12O7(aq) (a) What change in the ratio of atoms present indicates that the conversion of glucose into a compound with molecular formula (C6H12O7) is an example of oxidation? (a) The increase in the oxygen content indicates oxidation. (b)(i) The first titration result is a rough titration value and is not an accurate titre. (ii) No of moles = C x V(litres) = 0.5 x 0.0172 = 0.0086 From equation 1 mole of glucose reacts with 2 moles of copper(II) ions. So No, of moles of glucose = 0.0043. Concentration = 0.0043/0.025 = 0.172 mol l-1. (b) In one experiment, 25.0 cm3 volumes of a soft drink were titrated with Benedict’s solution in which the concentration of copper(II) ions was 0.500 mol l-1. The following results were obtained. Titration Volume of Benedict’s solution / cm3 1 18.0 2 17.1 3 17.3 Average volume of Benedict’s solution used = 17.2 cm3. (i) Why was the first titration result not used in calculating the average volume of Benedict’s solution? (ii) Calculate the concentration of glucose in the soft drink, in mol l-1. 5. Vitamin C, C6H8O6, is a powerful reducing agent. The concentration of vitamin C in a solution can be found by titrating it with a standard solution of iodine, using starch as an indicator. The equation for the reaction is: C6H8O6 (aq) + I2(aq) C6H6O6 (aq) + 2H+(aq) +2I–(aq) (a) Write an ion-electron equation for the reduction half-reaction. (b) What colour change indicates that the end-point of the titration has been reached? (c) In one investigation, it was found that an average of 29.5 cm3 of 0.02 mol l-1 iodine solution was required to react completely with 25 cm3 of vitamin C solution. Use this result to calculate the mass, in grams, of vitamin C present in the solution. (a) I2 + 2e- 2I – (b) A blue-black colour appears. (c) No. of moles of iodine used = C x V(litres) = 0.02 x 29.5/ 1000 = 5.9 x 10-4 mol From equation 1 mole of vitamin C reacts with 1 mole of iodine So number of moles of vitamin C is 5.9 x 10-4 mol. gfm of C6H8O6 = 176 g Mass = no. of moles x gfm = 5.9 x 10-4 = 0.104 g x 176











