1117747Notes 15.2B
advertisement
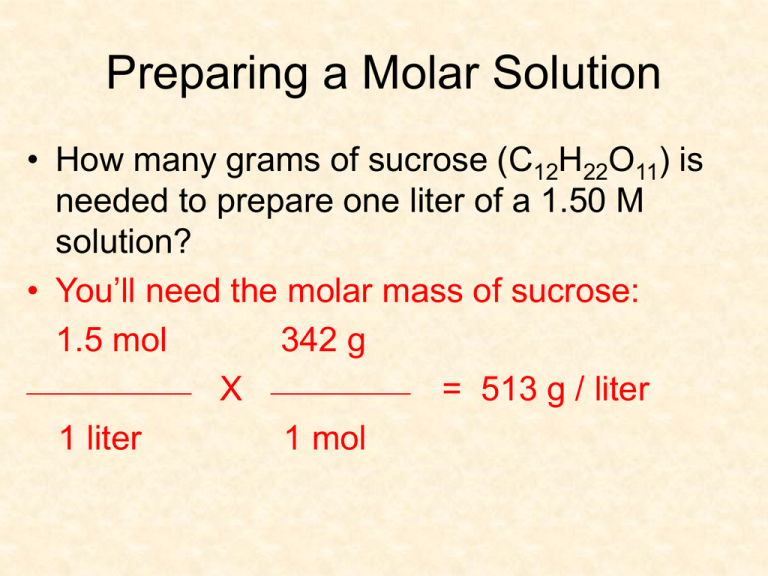
Preparing a Molar Solution • How many grams of sucrose (C12H22O11) is needed to prepare one liter of a 1.50 M solution? • You’ll need the molar mass of sucrose: 1.5 mol 342 g _____________ X ___________ = 513 g / liter 1 liter 1 mol Preparing a Molar Solution • How many grams of sucrose (C12H22O11) is needed to prepare 100 mL of a 1.50 M solution? 1.5 mol 342 g _____________ X ___________ = 513 g / liter 1 liter 1 mol 1L 100 mL X __________ 1000 mL 513 g X _____________ 1L = 51.3 g Diluting Solutions • Many solutions (acids) come in a concentrated liquid form called a stock solution • To dilute this solution, you add more solvent (usually water or alcohol) • The amount of solute does not change, so the amount of solution SO M1V1 = M2V2 Diluting Solutions • What volume in milliliters of 2.00M calcium chloride (CaCl2) would you use to make .50 L of .300 M calcium chloride solution? .50 L X 1000 mL/1 L = 500 mL M1V1 = M2V2 2.00M x V1 = .300M x 500 mL V1 = .300M x 500 mL / 2.00M V1 = 75 mL Mole Fraction • Another way to express concentration • Need to know the number of moles of solute and solvent • Abbreviated as X Xa = mole fraction of solvent = na / na + nb Xb = mole fraction of solute = nb / na + nb Mole Fractions • What is the mole fraction of water in an HCl solution containing 62.5% water and 37.5% HCl by mass? 62.5 g x 1mol / 18.02 g = 3.47 mol H2O 37.5 g x 1mol / 36.46 g = 1.03 mol HCl na / na + nb 3.47 mol H20 / 1.03 mol HCl + 3.47 mol H20 na = .771











