Ch11 Sample Problem #35
advertisement
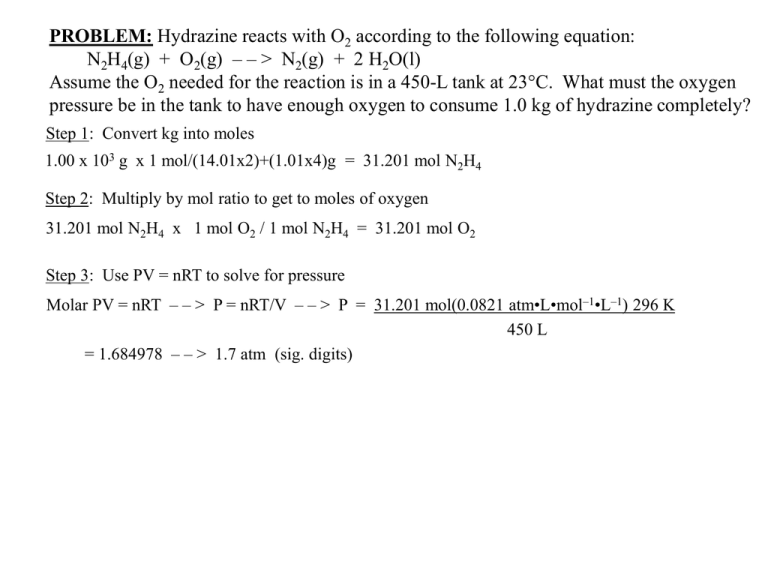
PROBLEM: Hydrazine reacts with O2 according to the following equation: N2H4(g) + O2(g) – – > N2(g) + 2 H2O(l) Assume the O2 needed for the reaction is in a 450-L tank at 23°C. What must the oxygen pressure be in the tank to have enough oxygen to consume 1.0 kg of hydrazine completely? Step 1: Convert kg into moles 1.00 x 103 g x 1 mol/(14.01x2)+(1.01x4)g = 31.201 mol N2H4 Step 2: Multiply by mol ratio to get to moles of oxygen 31.201 mol N2H4 x 1 mol O2 / 1 mol N2H4 = 31.201 mol O2 Step 3: Use PV = nRT to solve for pressure Molar PV = nRT – – > P = nRT/V – – > P = 31.201 mol(0.0821 atm•L•mol–1•L–1) 296 K 450 L = 1.684978 – – > 1.7 atm (sig. digits)











