Water Structure and Acid
advertisement

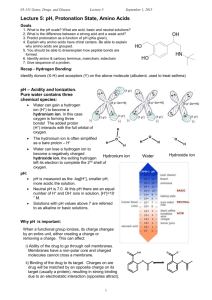
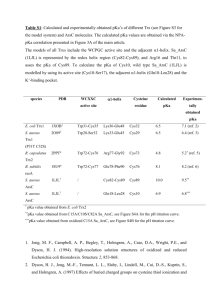
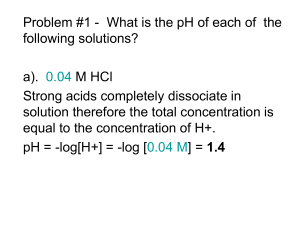
Biochemistry I Lecture 3 January 18, 2013 Solvation – Continued: ii) Hydrophilic (polar) compounds (e.g. methanol): H3C O H iii) Hydrophobic (apolar) compounds (e.g. methane). http://chem.ps.uci.edu/~kcjanda/Group/Re search_hydrates.html O iv)Amphipathic (or amphiphilic) compounds are both polar (usually charged) and have a substantial nonpolar section (e.g. fatty acids). These can form micelles if the nonpolar part is sufficiently large. Micelles are aggregates of amphipathic molecules that sequester the nonpolar part on the inside, much like the inside of an orange. O Lecture 3: Acid-Base Equilibria OLI Quiz: Q1 Readings: Horton: 2.7-2.9. Nelson & Cox: Chapter 2.2. Key Terms: Equilibrium constant Acid dissociation constant pH = pKa + log[A-]/[HA] Calculation of degree of protonation. Acid strength & chemical structure. Why pH is important in Biochemistry. i) Molecular interactions can be sensitive to pH. Changing the pH can change the charge on molecules. ii) Biological activity can be sensitive to pH. e.g. ionized groups may be required for function. 1 Biochemistry I Lecture 3 January 18, 2013 3A. Acids and Bases: pH: pH is measured as the -log[H+], smaller pH, more acidic the solution. Neutral pH is 7.0. At this pH there are an equal number of H+ and OH- ions in solution. [H+]=10-7 M. Acid: can donate protons to water, forming its conjugate base and an hydronium ion. Base: can accept protons The following describes ionization or dissociation of the proton from the acid. HA H 2O A H 3O acid + water conjugate + hydronium base Monoprotic acid: releases one proton (Acetic acid) Diprotic acid: releases two protons (Malonic acid) O Triprotic acid: releases three protons (Phosphoric acid) 3B. Characterization of Acid Strength Using pKa. When a proton dissociates from an acid, it becomes bound to a water molecule, generating a hydronium ion, in addition to the deprotonated acid: pKa OH O OH HO Since the concentration of water is essentially constant, it can be ignored and we can write a modified equilibrium reaction that just focuses on the species of interest: pKa1 OH OH pKa1 HA → A- + H+ and write the equilibrium constant for the dissociation: This is given a special name, the 'k-a', or 'k-acidity'. The acidity constant, Ka is a fundamental property of the acid, it does not depend on the pH of the solution. However, it does depend on the chemical structure and environment of the acidic group. 3C. pKa: Since the pH scale is used to characterize [H+], it is useful to express the acidity constant in the same way, by taking its negative log, giving the “p-K-a”: pKa = - log Ka Note: When the pH = pKa, 50% of the acid is protonated. 3D. Acid Strength and pKa The stronger the acid the more the reaction: HA → A- + H+ is to the right. How does the KA & pKa vary as the acid strength increases? 𝐾𝑎 = 2 O OH [𝐴− ][𝐻 +] [𝐻𝐴] O O pKa2 O HO O HO P O P O O O HO HA +H2O → A- + H3O+ O O O HO O O P pKa2 O O O O pKa3 P O O Biochemistry I Lecture 3 January 18, 2013 3E. Prediction of Protonation State at any pH: In many cases only one of the two species (protonated or deprotonated) may be biologically active. Given the pKa of the ionizable group, and the pH of the solution, we would like to calculate the following: The fraction that is protonated: fHA. The fraction that is deprotonated: fABeginning with the equilibrium constant for ionization: [ H ][ A ] [ HA] Take –log of both sides: f HA Ka [ H ][ A ] log K a log [ HA ] [ A ] log K a log[H ] log [ HA] [ A ] pK a pH log [ HA ] Defining [ HA] [ HA] [ A ] 1 [ A ] 1 [ HA] R f HA [A ] [ HA] 1 (1 R ) f A [ A ] [ HA] [ A ] [ A ] f A [ HA] 1 [A ] [ HA] R (1 R ) R=[A-]/[HA] pH pK a log([ A ] /[ HA]) pH pK a log( R) Example: Calculate the fraction protonated of the side chain of Histidine, an amino acid found in proteins. pKa = 6.0. pH pK a log( R) 10( pH pK a ) R Fraction Protonated H N + N H N + + N H H pH R=10(pH-pKa) FHA=1/(1+R) 4 R = 10 (4 - 6) = 10-2 FHA = 1/(1 + 0.01) = 0.99 5 R = 10 (5 - 6) = 10-1 FHA = 1/(1 + 0.10) = 0.91 6 R = 10 (6 - 6) = 100 FHA = 1/(1 + 1) = 0.5 7 R = 10 (7 - 6) = 10+1 FHA = 1/(1 + 10) = 0.091 8 R = 10 (8 - 6) = 10+2 FHA = 1/(1 + 100) = 0.01 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 1 2 3 4 5 6 7 8 9 10 11 12 pH 3 Biochemistry I Lecture 3 January 18, 2013 3F. Chemical Structure and Acidity: Key Concepts: The strength of an acid depends on the ability to break the A-H bond, e.g. NH versus OH. For a given type of acid, the strength depends on the relative stability of HA and A-, which can be affected by the chemical groups nearby and the environment. H N + H N H Amine, pKa ~10.7 H H Easier to break an N-H bond versus an O-H bond, therefore an amine is a stronger acid than an alcohol. Ethanol H O pKa ~14 O (Ser,Thr sidechain) H H H O H H H pKa ~ 4.0 O (Glu,Asp sidechain) F O F H O F F Acetic Acid O F H O H + H N H O H F pKa ~0 O H + H N H O Trifluoroacetic acid O Negative charge delocalized over C=O, lower in energy, therefore a carboxylate is a stronger acid than an alcohol. Electronegative fluorine atoms withdraw negative charge from carboxyl group, a stronger acid than acetic. O O Glycine (pKa =2.0) (an amino acid) Practice: 1. Assume that the histidine containing protein also contains a group that ionizes with a pKa of 4, draw the curve of fraction protonated versus pH for that group on the same graph. The curve for the histidine is already plotted for you. Fraction Protonated 2. Which region of the plot corresponds to both groups being fully deprotonated. 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 1 2 3 4 5 6 7 8 9 10 11 12 pH 4