Naming Acids and Bases
advertisement

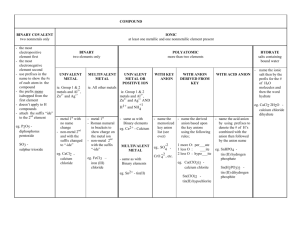
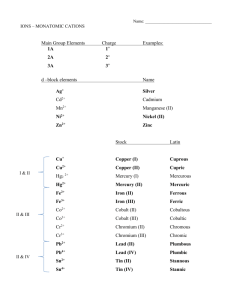
Acids: ◦ a compound that contains one or more hydrogen atoms and produces hydrogen ions (H+). Bases: ◦ An ionic compound that produced a hydroxide ion (OH-) when dissolved in water. Three Rules: ◦ 1) When the name if an the anion end in –ide, the acid name begins with hydro-. The end of the name has the suffix –ic and is following by the word acid. ◦ Example: HCl The anion is chloride goes to chloric Begins with hydro-. Goes to hydrochloric Followed by the word acid. Goes to hydrochloric acid. HCl is hydrochloric acid. 2) When the anion name ends in –ite, the acid name will end in –ous, followed by the word acid. ◦ Example: H2SO3 The anion is sulfite (SO3) The anion sulfite goes to sulfurous Followed by the word acid. Sulfurous acid H2SO3 is sulfurous acid. 3) When the anion name ends in –ate, the acid name changes the suffix to –ic, and it is followed by the word acid. ◦ Example: HNO3 The anion is nitrate. The anion nitrate goes to nitric Followed by the word acid. Nitric acid. HNO3 is nitric acid. Bases are named in the same way as other ionic compounds– the name of the CATION is following by the name of the ANION. ◦ Example: NaOH is sodium hydroxide Give the names of these acids: ◦ HNO2 ◦ H2S ◦ H2CrO4 ◦ Give the names of these bases: LiOH Mg(OH)2 Pb(OH)2 Al(OH)3 Give the names of these acids: ◦ HNO2 ◦ H2S ◦ H2CrO4 Nitrous Acid Hydrosulfuric Acid Chromic Acid ◦ Give the names of these bases: LiOH Mg(OH)2 Pb(OH)2 Al(OH)3 Work on study guide as a test review Q & A with Mrs. Branum Ask and you shall receive! If you do not work on study guide or ask questions, I WILL give you work to do. I have another assignment ready.