Naming Non-ionic Compounds
advertisement

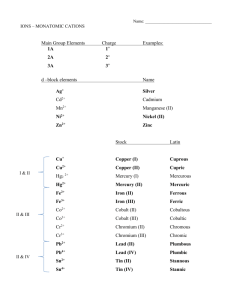
Naming Non-ionic Compounds Rules to Live By Rule #1 • The first element in the formula is named first, and the full element name is used. Rule #2 • The second element is named as it would be if it was an anion. – Ex. Cl would be “chloride” like before. Rule #3 • Prefixes are used to tell the number of atoms in the formula. – We will look at these in 2 more slides. Rule #4 • The prefix mono- (meaning 1) is never used for the first element. – Ex. CO2 is carbon dioxide, not monocarbon dioxide. Ever. Prefixes 1 mono- 2 3 4 5 6 7 8 ditritetrapentahexaheptaocta- Yay Practice! Name the following compounds: 1. PCl5 Phosphorus pentachloride 2. P4O6 Tetraphosphorus hexoxide 3. SF6 Sulfur hexafluoride 4. SO3 Sulfur trioxide 5. SO2 Sulfur dioxide 6. N2O3 Dinitrogen trioxide Now go the other way! • 1. 2. 3. 4. 5. Give the formula for the following compounds! Carbon pentasulfide CS5 Diphosphorus hexachloride P2Cl6 Hexanitrogen monofluoride N6F Tetroxygen trichloride O4Cl3 Phosphorus pentachloride PCl5 Let’s Do Acid! In the learn-how-to-name-them sense… Naming Acids 1. If the anion does not contain oxygen, the acid is named with the prefix hydro- and the suffix -ic. Acid HCl HCN H 2S Anion ClCNS-2 Name . hydrochloric acid hydrocyanic acid hydrosulfuric acid Naming Acids 2. When the anion contains oxygen, we add either –ic or –ous to the end. -ic we use when we have an anion whose name ends in –ate: Acid H2SO4 H3PO4 HC2H3O2 Anion sulfate phosphate acetate Name . sulfuric acid phosphoric acid acetic acid Naming Acids -ous we use when we have an anion whose name ends in –ite. Acid H2SO3 HNO2 Anion sulfite nitrite Name . sulfurous acid nitrous acid Acid (starts with H and is not water) The anion (-) name ends in… -ide Single element from the periodic table: Br, Cl, I, S… (root) “Hydro_______ic Acid” -ite -ate Only polyatomic ions Only polyatomic ions (root) “_____ous Acid” (root) Acid” “_____ic Practice! Name the following acids. Acid 1. HF 2. H3PO3 3. HNO3 4. H2S Anion Name . F- (fluoride) hydrofluoric acid PO3-3 (phosphite) phosphorous acid NO3- (nitrate) nitric acid hydrosulfuric acid S-2 (sulfide) The End Now let’s do some more practice.