Chem 151L, Spring 2015
advertisement

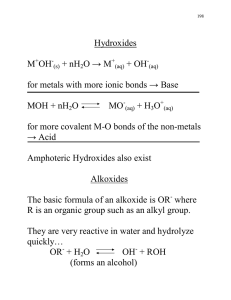
Chem 151L, Spring 2015 Expt. 2. Synthesis and Anion-Exchange of a Metal-Organic Framework Metal-organic frameworks (MOFs) are covalent networks where metal atoms or clusters are connected by organic linkers.1 These extended structures can be 1-D chains, 2-D layers or 3-D frameworks. The materials are of interest for a variety of potential applications including gas storage, sensors, catalysis and solid state batteries. An example is the MOF known as SLUG-21 (Figure 1), [Ag2(4,4’-bipy)22+][–O3SCH2CH2SO3–]·4H2O, where bipy is bipyridine, (C5H4N)2.2 In this lab, you will synthesize a related MOF known as silver bipyridine nitrate (SBN, [Ag(4,4’-bipy)+][NO3–]).3 This material is a rare example of a host material that possesses a charge. Nitrate anions reside between the layers and may be reversibly exchanged out of the material for other anions in solution including pollutants such as perchlorate, chromate and pertechnetate. Figure 1. SLUG-21 displays reversible, selective uptake of a variety of inorganic and organic anions. Left: side view of the cationic layers and + interlayer anions; right: view of one layer of -stacked Ag-bipy polymers. Week 1: Place a stir bar and 10 mL of distilled water in a beaker, followed by 0.10 g silver nitrate. With manual stirring, add 0.10 g of 4,4’-bipyridine. Allow to mechanically stir for at least 50 minutes, then parafilm the beaker and leave in your drawer until the following lab period. Week 2: At the start of the following lab period, filter the product, rinsing with acetone. Weigh the product and calculate the percent yield. Place ~ 50 mg of the product in a vial and label with your names, TA name and “as-synthesized SBN”. Place 80 mg of the product in a beaker containing 50 mL distilled water. Add 0.25 millimoles of the anion that has been assigned to you and your lab partner. Stir mechanically for at least 1 hour. Filter the product into a clean flask and analyze the liquid by FTIR or UVVis, depending on the anion you are studying. Place the solid in a labelled vial (names, TA name and “SBN-anion”, where “anion” is the anion formula) for PXRD analysis. Your TA will collect the powder X-ray diffraction (PXRD) data before the next lab period. Questions: 1) Write a balanced set of equations for the synthesis steps and determine the yield. 2) Closely compare the PXRD patterns before and after the exchange. If you have UVVis data, calculate the percent exchange. 3) Why was excess anion used for the exchange? 4) What other characterization techniques could be used to monitor the exchange if they were available? References 1) Zhou, H.-C.; Long, J. R.; Yaghi, O. M. Chem. Rev. 2012, 112, 673-674 and journal articles within that special issue on MOFs. 2) Fei, H.; Bresler, M. R.; Oliver, S. R. J. J. Am. Chem. Soc., 2011, 133, 11110–11113. 3) O. M. Yaghi, H. L., J. Am. Chem. 1996, 118, 295-296.