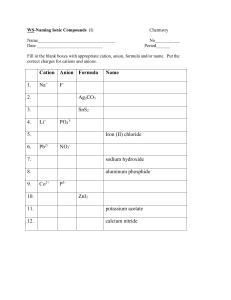
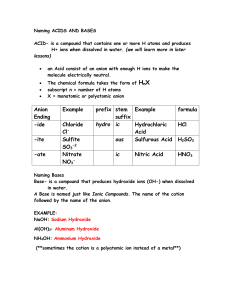
ACTIVITY 4. Use of Sets of Illustrative Examples In writing formulas of molecular compounds, use Greek prefix(es) to determine number of atoms of each element in formula. Get elements and number of atoms of each from name: Illustrative Examples Set 1: tetraphosphorus hexasulfide dibromoheptoxide P4S6 Br2O7 In writing formulas of acids, given an anion, we can get formula of acid by: adding H atoms equal to negative charge on ion Illustrative Examples Set 2: F= fluoride ion (add # of H's equal to negative charge) NO2– = nitrite ion (add # of H's equal to negative charge) SO4–2 = sulfate ion (add # of H's equal to negative charge) Formula: HF (aq) Formula: HNO2 (aq) Formula: H2SO4 (aq) In writing formulas and names of acids, given an anion, we can name for acid: depending on suffix of anion name. Illustrative Examples Set 3: 1. If anion name ends with “–ide”, add prefix hydro and change “–ide” to “–ic” and add the word acid. Example: HF (aq) (anion name for F-1 is fluoride ) HF (aq) = hydrofluoric acid 2. If anion name ends with “–ite”, change “–ite” to “–ous” and add the word acid. Example: HNO2 (aq) (anion name for NO2-1 is nitrite) HNO2 (aq)= nitrous acid 3. If anion name ends with “-ate”, change “-ate” to “-ic” and add the word acid. Example: H2SO4 (aq) (anion name for SO42- is sulfate) H2SO4 (aq)= sulfuric acid