Consumer Products
advertisement

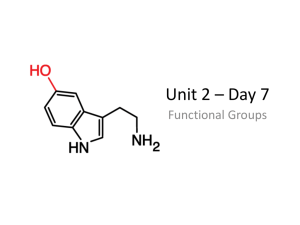
I know/I can… Everyday Consumer Products; 1. alcohols are a group of compounds containing the hydroxyl (-OH) functional group. 2. to write molecular formulae and draw structural formulae for straight chain alcohols given their name. 3. to name straight chain alcohols given their shortened or full structural formulae. 4. alcohols show a gradation in their physical properties. 5. alcohols are very good solvents. 6. alcohols burn with a very clean flame and can be used as fuels. 7. combustion, burning of fuels, is an exothermic process. 8. endothermic reactions take in energy from their surroundings. 9. fuels can be compared by measuring the energy given off when they are burned. 10. when a burning fuel is used to heat water the energy transferred is calculated using the formula Eh = cmΔT. 11. balanced equations can be used to calculate the masses of reactants and products of a reaction. 12. carboxylic acids are a homologous series of compounds containing the carboxyl (-COOH) functional group. 13. to write molecular formulae and draw structural formulae for straight chain carboxylic acids given their name. 14. to name straight chain carboxylic acids given their shortened or full structural formulae . 15. vinegar is a solution of ethanoic acid in water. 16. vinegar can be used as a preservative. 17. many household cleaners contain vinegar. 18. esters are made by reacting alcohols with carboxylic acids. 19. esters are used as food flavourings and as solvents.