Document
advertisement

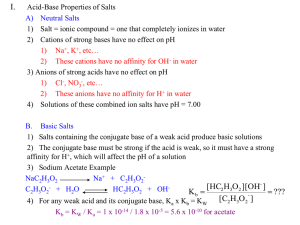
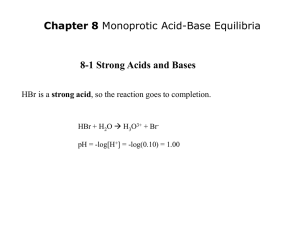
Additional Aspects of Aqueous Equilibria Chapter 17 The Common Ion Effect and Solubility The presence of a common ion decreases the solubility of the salt AgBr (s) Ag+ (aq) + Br- (aq) If we add some solid NaBr to this solution: NaBr (s) Na+ (aq) + Br- (aq) Buffered solution ≡ solution has the ability to resist changes in pH upon the addition of small amounts of either acid or base. Consists of: 1. A weak acid or a weak base and 2. The salt of the weak acid or weak base Both must be present! Fig 17.2 If a small amount of hydroxide is added to an equimolar solution of HF in NaF, for example, the HF reacts with the OH− to make F− and water Aqueous Equilibria © 2009, Prentice-Hall, Inc. Fig 17.2 Similarly, if acid is added, the F− reacts with it to form HF and water Aqueous Equilibria © 2009, Prentice-Hall, Inc. Which of the following are buffer systems? (a) KF/HF (b) KBr/HBr, (c) Na2CO3/NaHCO3 (a) KF is a weak acid and F- is its conjugate base buffer solution (b) HBr is a strong acid not a buffer solution (c) CO32- is a weak base and HCO3- is it conjugate acid buffer solution What is the pH of a solution containing 0.30 M HCOOH and 0.52 M HCOOK? Ka for HCOOH = 1.8 X 10-4. Mixture of weak acid and conjugate base! HCOOH (aq) Initial (M) Change (M) Equilibrium (M) H+ (aq) + HCOO- (aq) 0.30 0.00 0.52 -x +x +x 0.30 - x x 0.52 + x Can we approximate? Is [HCOOH]o > 100 Ka? YES! 0.30 – x 0.30 0.52 + x 0.52 x = [H+] = 1.04 X 10-4 M pH = 3.98 Consider mixture of salt NaA and weak acid HA. NaA (s) HA (aq) [H+] Na+ (aq) + A- (aq) H+ (aq) + A- (aq) Ka [HA] ≈ [A-] -log [H+] ≈ -log Ka - log [H+][A-] Ka = [HA] Henderson-Hasselbalch [HA] [A-] -] [A -log [H+] ≈ -log Ka + log [HA] [A-] pH ≈ pKa + log [HA] Equation [conjugate base] pH ≈ pKa + log [acid] pKa = -log Ka What is the pH of a solution containing 0.30 M HCOOH and 0.52 M HCOOK? Ka for HCOOH = 1.8 X 10-4. Mixture of weak acid and conjugate base! HCOOH (aq) H+ (aq) + HCOO- (aq) [HCOO-] pH ≈ pKa + log [HCOOH] HCOOH pKa = 3.74 [0.52] pH ≈ 3.74 + log = 3.98 [0.30] Limitations in Using HendersonHasselbalch Equation • H-H equation is an approximation • Fails when [HA] < 100 Ka or [BH] < 100 Kb • pH of buffer made ≠ pH calculated • Uncertainty in Ka and in Kb • Approximations made (no use of quadratic eqn.) • Ion-ion interactions when M > 0.01 M (no activities) Buffer capacity ≡ the number of moles of a strong acid or strong base that causes 1.00 L of a buffer to change pH ± 1.00 unit. [conjugate base] pH ≈ pKa + log [acid] pKa for the acid should lie within ± 1 unit of desired pH Want: log [NaA] [HA] 0 i.e., [HA] ≈ [NaA] Chemistry In Action: Maintaining the pH of Blood Fig 17.4 Red blood cells pH = 7.35 – 7.45 Carbonate- bicarbonate buffer EXAM #4 • Covers Chapters 16 and 17.1 – 17.2 • 40 Multiple Choice (80%, all qualitative) • Two calculations: (20%) • Calculate pH of a weak acid solution • Calculate pH of a buffer • All equations provided • Constants & Periodic Table provided Practice problems: 1) Calculate the pH of a 0.060 M HF solution. Ka = 7.1 x 10-4. 2) What is the pH of a buffer of 0.15 M NH3 / 0.35 M NH4Cl? Kb for NH3 = 1.8 x 10-5.