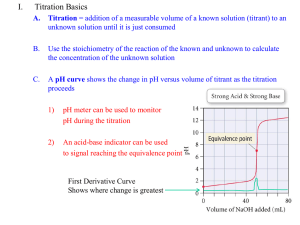
BL base
advertisement

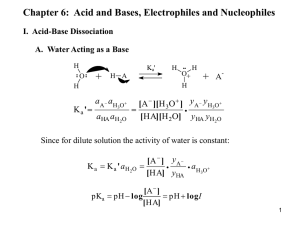
CH4. Acids and Bases 1 Bronsted-Lowry Bronsted-Lowry definitions: Acid = proton donor; Base = proton acceptor HF (aq) + H2O BL acid H3O+ (aq) + F- (aq) BL base Fluoride ion is the conjugate base of HF Hydronium ion is the conjugate acid of H2O 2 Amphiprotic species Amphiprotic – species that can act as BL acid or base NH3 (aq) + BL base H2O BL acid NH4+ (aqu) + OH (aqu) hydroxide Kb = base dissociation constant = [NH4+] [OH] / [NH3] H2O is amphiprotic - it’s a base with HF, but an acid with NH3 3 BL acid/base strength Ka, the acidity constant, measures acid strength as: Ka = [H3O+] [A-] / [HA] pKa = - log Ka When pH = pKa, then [HA] = [A-] For strong acids pKa < 0 pKa(HCl) ≈ -7 4 BL acid/base strengths 5 Kw Kw = water autodissociation (autoionization) constant 2 H 2O H3O+ (aqu) + OH- (aqu) Kw = [H3O+] [OH-] = 1 x 10-14 (at 25°C) Using the above, you should prove that for any conjugate acid-base pair: pKa + pKb = pKw = 14 6 Polyprotic acids Since pKa values are generally wellseparated, only 1 or 2 species will be present at significant concentration at any pH H3PO4 + H2O H2PO4- + H3O+ pKa1 = 2.1 H2PO4- + H2O HPO42- + H3O+ pKa1 = 7.4 HPO42- + H2O PO43- + H3O+ pKa1 = 12.7 7 Solvent leveling The strongest acid possible in aqueous solution is H3O+ Ex: HCl + H2O H3O+ (aq) + Cl- (aq) there is no appreciable equilibrium, this reaction goes quantitatively; the acid form of HCl does not exist in aqueous solution Ex: KNH2 + H2O K+ (aq) + OH- (aq) + NH3 (aq) this is solvent leveling, the stable acid and base species are the BL acid-base pair of the solvent NH2- = imide anion NR2- , some substituted imide ions are less basic and can exist in aq soln 8 Solvent leveling Only species with 0 < pKa < 14 can exist in aqueous solutions. The acid/base range for water stability pKw, i.e. 14 orders of mag in [H+]. Other solvents have different windows and different leveling effects. 9 Solvent leveling 2EtOH EtOH2+(solv) + EtO (solv) K ~ 1020 chemistry in the range of -3 < pKa < 17 NH3 NH4+(solv) + NH2(solv) ammonium imide chemistry in the range of 10 < pKa < 38 O2 OH NH3(l) Na (m) Na+ (solv) + NH2(solv) + ½ H2 (g) slow very strong base Na+ (solv) + e (solv) 10 Acid/base chemistry of complexes Aqueous chemistry: H2O Fe(NO3)3 [Fe(OH2)6]3+(aq) + 3 NO3(aq) 2 [Fe(OH2)6]3+ (aq) Hexaaquairon(III), pKa ~ 3 [Fe2(OH2)10OH]5+ (aq) + H3O+(aq) dimer 11 Aqua, hydroxo, oxoacids aqua acid M(OH2)xn+ ex: [Cu(OH2)6]2+ hydroxoacid M(OH)x ex: B(OH)3 , Si(OH)4 pKa ~ 10 oxoacid MOp(OH)q p and q designate oxo and hydroxo ligands hexaaquacopper(II) cation ex: H2CO3 (aq) + H2O HCO3 (aq) + H3O+(aq) carbonic acid CO2 (g) + H2O bicarbonate pKa ~ 3.6 12 Trends in acidity For aqueous ions: pKa vs z2 / (r++ d) 1. Higher charge is more acidic pKa of [Fe(OH2)]3+ ~ 3 pKa of [Fe(OH2)]62+ ~ 9 2. Smaller radius is more acidic Mn2+ Cu2+ early TM late TM lower Z* higher Z* => larger radius => smaller radius less acidic more acidic Na+ (aqu) = [Na(OH2)6]+ has pKa > 14 so it’s a spectator ion in aqu soln 13 Anhydrides Ex: H2O + SO3 anhydride H2SO4 acid form Acidic SO3 / H2SO4 “P2O5” / H3PO4 CO2/H2CO3 Basic Na2O / NaOH Amphoteric Al2O3 / Al(OH)3 14 Trends in acidity 15 Common acids HNO3 NO3 (D3h) Nitric acid Nitrate HNO2 NO2 (C2v) Nitrous acid Nitrite H3PO4 PO43 (Td) Phosphoric acid Phosphate H3PO3 HPO32 (C3v) Phosphorous acid Phosphite You should know these! 16 Common acids H2SO4 (Td) SO42 Sulfuric acid Sulfate H2SO3 (C3v) SO32 Sulfurous acid Sulfite You should know these! 17 Common acids HClO4 ClO4 (Td) Perchloric acid Perchlorate HClO3 ClO3 (C3v) Chloric acid Chlorate HClO2 ClO2 (C2v) Chlorous acid Chlorite HOCl OCl Hypochlorous acid Hypochlorite You should know these! 18 Pauling’s rules for pKa‘s of oxoacids 1. Write formula as MOp(OH)q 2. pKa 8 – 5p 3. Each succeeding deprotonation increases the pKa by 5 Ex: rewrite HNO3 as NO2(OH) p = 2; pKa 8 – 5(2) 2 (exptl value is 1.4) Ex: rewrite H3PO4 as PO(OH)3 p = 1; pKa1 8 – 5(1) 3 (exptl value is 2.1) pKa2 8 (exptl value is 7.4) pKa3 13 (exptl value is 12.7) 19 pKa values p Pauling pKa calcn exptl Cl(OH) 0 8 7.5 ClO(OH) 1 3 2.0 ClO2(OH) 2 2 1.2 ClO3(OH) 3 7 ≈ 10 HlO4 + 2H2O H5IO6 20 Amphoteric oxides [Al(OH2)6]3+ Oh Al2O3 / Al(OH)3 [Al(OH)4] H3O+ OH Td 2 [Al(OH2)6]3+(aq) pKa ~ 2 [Al2(OH2)10(OH)]5+(aq) + H3O+(aq) dimer 21 polyoxocations linear trimer is [Al3(OH2)14(OH)2]7+ Keggin ion [AlO4(Al(OH)2)12]7+ pH ≈ 4 charge/volume ratios Al(OH2)63+ 3+ / Oh > dimer 5+ / 2 Oh > trimer 7+ / 3 Oh --- > Al(OH)3 neutral 22 Polyoxoanions VO43(aq) H3O+ V2O5(s) orthovanadate (Td) 2 VO43(aq) + H2O V2O74 (aq) + 2OH (aq) H3O+ V3O93 V3O105 H3O+ oxo bridge V4O124 23 Lewis acids and bases A LA + B: LB A:B complex LA = electron pair acceptor; LB = electron pair donor Lewis definition is more general than BL definition, does not require aqueous or protic solvent Ex: W + 6 :CO [W(CO)6] BCl3 + :OEt2 BCl3:OEt2 D3h Fe3+(g) + 6 :OH2 → [Fe(OH2)6]3+ 24 LA/LB strengths LA strength is based on reaction Kf LA/LB strengths depend on specific acid base combination Ex: BCl3 + :NR3 Kf: NH3 < MeNH2 < Me2NH < Me3N BMe3 + :NR3 Kf: Cl3B:NR3 Me3B:NR3 NH3 < MeNH2 < Me2NH > Me3N Hrxn 58 74 inductive effect 81 inductive + steric 74 kJ/mol 25 log K and ligand type 26 Drago-Wayland equation A (g) + :B (g) A:B (g) Gas phase reactions (omits solvation effects) -Hrxn = EA EB + CA CB look up E, C values for reactants (Table 4.4) 27 Donor/Acceptor numbers Commonly used to choose appropriate solvents (Table 4.5) Donor Number (DN) is derived from Hrxn (SbCl5 + :B Cl5Sb:B) higher DN corresponds to stronger LB Acceptor Number (AN) is derived from stability of Et3P=O:A complex higher AN corresponds to stronger LA Ex: THF (tetrahydrofuran) C4H8O DN AN ε dielectric constant THF 20 8 7 H2O 18 55 82 Some Li+ salts and BF3 have similar solubilities in THF, H2O NH3 is much more soluble in H2O Most salts are much more soluble in H2O 28 Descriptive chemistry - Group 13 Expect inductive effect ex: BF3 > BCl3 > BBr3 but the opposite is true BF3 is stable in H2O, R2O (ethers) BCl3 rapidly hydrolyzes due to nucleophilic attack of :OH2 the lower acidity of BF3 is due to unusually favorable B–X bonding in the planar conformation due to interaction “AlCl3“ is a dimer (Al2Cl6) General trend larger central atom, tends to have higher CN Al2Me6 is isostructural with Al2Cl6 Friedel-Crafts C6H6 C6H5C(O)R RC(O)-X: + “AlCl3” RC(O) + AlCl3X 29 Descriptive chemistry - Group 14 CX4 is not a Lewis Acid Acidity SiF4 > SiCl4 > SiBr4 > SiI4 (inductive effect) ex: 2KF(s) + SiF4(g) K2SiF6(s) LB LA SiF62 Oh SnF4 and PbF4 have Oh not Td coordination (heavier congener, higher CN) each M has 2 unique axial F and 4 shared F 30 Descriptive chemistry - Group 15 MF5 does not exist for nitrogen; it’s trigonal bipyramidal for M = P, As SbF5: Sb has Oh coordination (oligomerizes to Sb4F20 or Sb6F30) LB LA K2MnF6 (s) + 2 SbF5 (l) KF, H2O2 aqu HF KMnO4 Sb2O3 transient “MnF4” + 2KSbF6 (s) F transfer MnF3 + ½ F2 (g) Dove (1980’s), chemical synthesis of F2 gas 31 Descriptive chemistry - Group 16 Inductive effect stabilizes conjugate base (anionic form) sulfuric acid fluorosulfonic HSO3F / SbF5 pKa ~ 2 pKa ~ 5 pKa ~ 26 (superacid) C6H6 HSO3F / SbF5 C6H7+ SbF6 32