Chirality & Enantiomers: sp3 Hybridized Systems Worksheet
advertisement

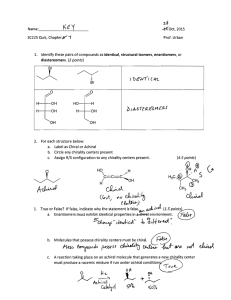
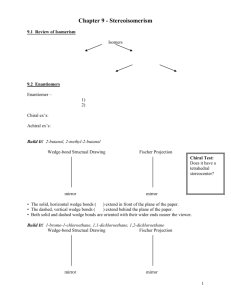
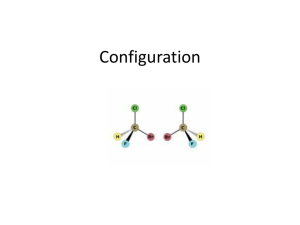
sp3 hybridized systems: chirality and enantiomers Major concepts sp3 hybridization leads to a tetrahedral shape with geometry in three dimensions Chirality is a property of a molecule which is not superimposable on its mirror image Enantiomers are related by the fact that they are non-superimposable mirror images of each other The absolute stereochemistry of a compound can be determined and labeled as R or S Vocabulary Tetrahedral center Chirality center Chiral, achiral Enantiomers Absolute stereochemistry Designated and undesignated chirality centers Students should be able to: Characterize a compound as chiral or achiral using A. chirality centers or B. nonsuperimposable mirror image Recognize the relationship of compounds as “same identity”, “enantiomers”, or “constitutional isomers” Draw the enantiomer of a compound Label a chirality center as R or S Daily Problems 1. Organic as a second Language Chapter 7 problems 7.1-7.43, 7.51-7.56 2. The chirality centers in the following molecules are not designated. Indicate all chirality centers with an asterisk (*). If there are no chirality centers, then indicate that this is the case. * * none none * * none none 3. Label the relationship of each of these molecules as “same”, “enantiomer”, or “constitutional isomer.” Constitutional isomers same same Enantiomers Enantiomers Enantiomers Cumulative problems 4. Draw structures for these compounds. If they are chiral compounds, give the name of the enantiomer. A. (1S,2S)-1,2-dimethylcyclohexane Enantiomer: (1R,2R)-1,2-dimethylcyclohexane B. (R)-3-chloropentan-1-amine Enantiomer: (S)-3-chloropentan-1-amine C. (S)-5-methylhex-4-en-3-ol Enantiomer: (R)-5-methylhex-4-en-3-ol 5. Draw the following structures, and indicate if any of the carbon atoms are Lewis acidic or Lewis Basic. (Always consider resonance!) A. 4-methylpent-3-en-2-one B. 4-methylpent-4-en-2-one C. (S)-3-bromo-4-phenylbutanal 6. What is the relationship of these two structures? These are resonance structures. Extension problems 7. Draw a resonance structure for this compound. Label the hybridization of the nitrogen atom in both cases. What is the actual hybridization??? δ+ δ+