Oxidation of Toluene: Lab Experiment & Aromatic Chemistry
advertisement
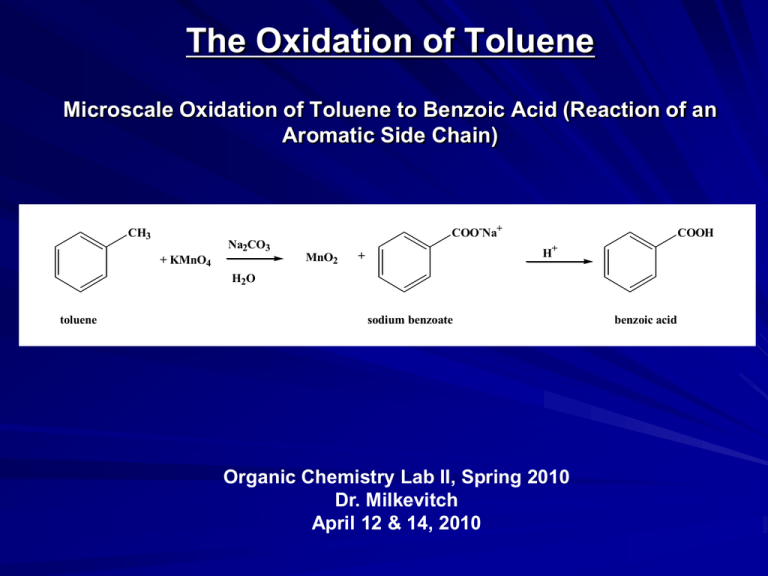
The Oxidation of Toluene Microscale Oxidation of Toluene to Benzoic Acid (Reaction of an Aromatic Side Chain) COO-Na+ CH3 Na2CO3 MnO2 + KMnO4 COOH H+ + H2O toluene sodium benzoate Organic Chemistry Lab II, Spring 2010 Dr. Milkevitch April 12 & 14, 2010 benzoic acid Today’s Experiment Conduct an oxidation of a group attached to an aromatic ring – Convert an alkyl group on an aromatic ring to a carboxylic acid group Purpose of the experiment – Review aromatic compounds Their structure, stability, reactivity – Demonstrate an oxidation of an aromatic side chain Selectively oxidize a group on an aromatic ring Leaving the aromatic ring intact (unoxidized) Reactions of Benzene, (con’t) There are many reactions of benzene – Last week: examined electrophilic aromatic substitution Today: Oxidize aromatic side chain Remember: – Benzene does not react like a conjugated cyclic triene – You can oxidize an alkene, but not a benzene ring With potassium permanganate H H OH KMnO4, H2O H OH H KMnO4, H2O no reaction Oxidation of Benzene Side Chains Benzene ring resistant to oxidation – By strong oxidants like potassium permanganate – Not so with alkyl side chains on aromatic rings Reactions take place at benzylic carbons – Benzylic carbon must have at least one hydrogen – Intermediates: benzylic radicals or benzylic carbocations – Mechanism not well understood This Experiment Oxidant: potassium permanganate Substrate is toluene COO-Na+ CH3 Na2CO3 MnO2 + KMnO4 COOH H+ + H2O toluene sodium benzoate benzoic acid Cautions!!! Potassium permanganate – Strong oxidizer – Avoid contact! – Will stain skin and clothing Its seriously purple! Procedure I Into a 10 ml RB flask, place: – – – – 825 mg of potassium permanganate 100 mg of sodium carbonate 3 ml of water Small spin bar Attach reflux condenser to flask Attach heating mantle Dissolve reagents with gentle stirring/heating Turn off heat, let cool ~ 3 min Add 250 microliters of toluene – Approx 10 mg of detergent – Aids mixing of toluene and water Reflux with stirring for 45 min – Do not overheat, for it can “bump” Procedure II Most of the purple color should be gone by the end of the reflux – Turn off water to condenser While still warm: – Add sodium bisulfite until the purple color is gone – Filter off MnO2 using a Hirsch funnel containing Celite Celite is a filter aid Keeps filter paper from clogging See the lab TA or myself for Celite Rinse flask with 0.5 ml of ddH2O, add this to filtrate Check filtrate: – Filtrate is what you want! – If its still purple colored, add sodium bisulfite until the solution is colorless – Filter solution again if more brown precipitate forms Cool filtrate in ice Acidify with 1 ml of concentated HCl Collect crystals by vacuum filtration using a Hirsch funnel Wash crystals with 1 ml of cold water Recrystalize from water Dry crystals, weigh and take a melting point Formal lab report required