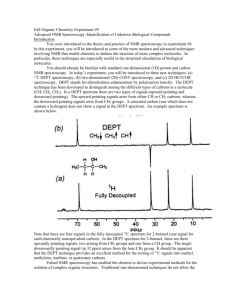
Spectroscopy Review
advertisement

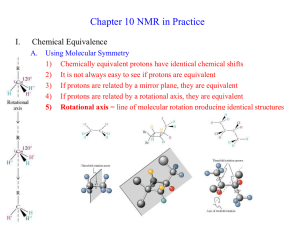
STRUCTURE DETERMINATION: MS, IR, NMR (A REVIEW) Dr. Sheppard CHEM 2412 Fall 2014 McMurry (8th ed.) sections 12.1-3, 12.5-8, 13.1-5, 13.7-11, 13.13 Spectroscopy • • • • Analytical techniques Help determine structure Destroy little or no sample Light absorbed by the sample is measured as wavelength varies • Types: 1. Mass spectrometry (MS) • Fragments the molecule and measures the masses 2. Infrared (IR) spectroscopy • Measures the bond vibration frequencies in a molecule and is used to determine the functional group 3. Nuclear magnetic resonance (NMR) spectroscopy • Number, type and connectivity of atoms in a molecule 4. Ultraviolet (UV) spectroscopy • Uses electron transitions to determine bonding patterns (conjugated p systems) MASS SPECTROMETRY Mass Spectrometry • Used with Gas Chromatography • Mixture of compounds separated by gas chromatography, then identified by mass spectrometry • Determines MW and provides information about structure • A beam of high-energy electrons breaks molecules into ions (fragments) M → M•+ + eM•+ → A+ + X A+ → B+ + Y etc. • Ions are separated and detected; mass determined The Mass Spectrum • Plot relative abundance vs. mass-to-charge ratio • Charge = +1 • Base peak = strongest (most abundant/stable ion) • Molecular ion/parent peak (M+) = mass of compound 14 14 15 Mass Spectrum of Hexane 14 14 14 15 Isotopes 81 Br • Present in their usual abundance • Hydrocarbons contain 1.1% 13C, so there will be a small M+1 peak • If S is present, M+2 will be 4% of M+ • If Cl is present, M+2 is one-third of M+ • If Br is present, M+2 is equal to M+ • If I is present, peak at 127; large gap INFRARED SPECTROSCOPY IR Spectroscopy • Units are wavenumbers (4000-400 cm-1) • Measures molecular vibrations • No two molecules will give exactly the same IR spectrum (except enantiomers) IR Spectrum Baseline Absorbance/ Peak • Simple stretching: 1500-4000 cm-1 • Complex vibrations: 400-1500 cm-1 • The “fingerprint region” • Interpretation: • Looking for presence/absence of functional groups • Correlation tables • Polar bonds is usually the most IR-active IR Correlation Table (McMurry 8th ed.) Hexane , Hexene and Hexyne Spectra Alcohol and Amine Spectra Carbonyl Spectra NMR NMR • Most powerful technique for structure determination • Number and type of atoms in a molecule • Connectivity of atoms • Used to study a wide variety of nuclei: • 1H, 13C, 15N, 19F, 31P, etc. • Radio-frequency radiation used to transition between energy states (nuclear spin) • Spinning nucleus acts as a bar magnet • Aligns with or against external field • Absorption of light causes spin flip • “Resonance” • Measured by spectrometer Nuclei in a Molecule • Depending on their chemical environment, atoms in a molecule are shielded by different amounts • Chemically equivalent nuclei • Interchanged through bond rotation or element of symmetry • Have same absorption • Chemically different nuclei have different absorption Chemical Shifts (d scale, in ppm) NMR Spectra TMS = Reference Compound 13C-NMR • Signal = one sharp line for each different type of carbon • The number of different signals indicates the number of different kinds of carbon • The chemical shift indicates the functional group • Used to support 1H-NMR analysis 1H-NMR • More info than 13C-NMR • The number of signals • How many different kinds of protons are present • The location (chemical shift) of the signals • Is the proton shielded or deshielded • The intensity (integration) of the signal • The number of protons of that type • Signal splitting (multiplicity) • The number of protons on adjacent atoms 1H-NMR Number of Signals • One signal for each type of H in a molecule 2 4 3 1H-NMR Chemical Shifts • More shielded = upfield (to the right) • Less shielded = downfield (to the left) 1H-NMR Integration • The signal intensity (area under signal) is proportional to the number of protons giving rise to that signal • Shown by integration line • Height of vertical line ≈ area under peak ≈ # H’s in set • Measure height with ruler or look at graph paper • Ratio of height = ratio of hydrogens 1H-NMR Spin-Spin Splitting • Signals can be split into multiple peaks • The (n+1) rule: • A signal is split by n neighboring protons, into (n + 1) peaks 1H-NMR Spin-Spin Splitting • Coupling constants (J) • Distance between the peaks of a split signal • Measured in Hz (usually 0-18) • Gives info on type of H Stereochemical Nonequivalence • Usually, two protons on the same carbon are equivalent and do not split each other • If the replacement of each of the protons of a -CH2 group with an imaginary “Z” gives stereoisomers, then the protons are non-equivalent and will split each other • Results in more complex splitting patterns a c CH c H H a H • Examples: OH C C 3 Hb d H H b H Cl aH Hb Cl Solving NMR Problems • Given: • 1H-NMR • Molecular formula (typically) • IR (sometimes) • 13C-NMR (sometimes) • Goal: Determine structure • If the molecular formula is known: • Determine the number of elements of unsaturation • Sum of number of rings + p bonds • Index of Hydrogen Deficiency IHD = C – ½(H + X) + ½N + 1 • Example: C5H3N2O2Cl IHD = 5 – ½(3 + 1) + ½(2) + 1 = 5