bio98a_l02
advertisement

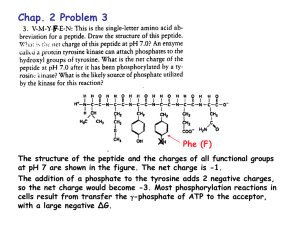
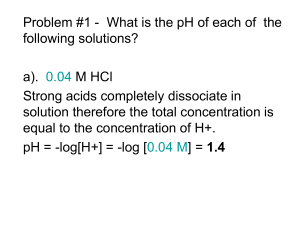
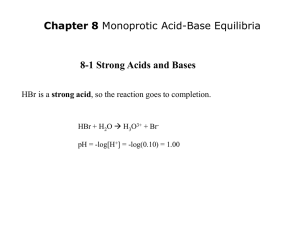
http://en.wikipedia.org/wiki/Acid_Queen Bio 98 - Lecture 2 Acid-Base Equilibria, pH and Buffers pH profiles of enzymatic reactions Amylase Pepsin UCI Bio199 Independent Research Pure water is only slightly ionized H2O H+ + OH- H+ ions (protons) do not persist free in solution, they are immediately hydrated to hydronium ions (H+ + H2O H3O+). Grotthuss proton wire Achieving equilibrium H2O H+ + OH- [H+] [OH-] Keq = = 1.8x10-16 M [H2O] Concentration of “water in water” ([H2O]) is 55.6 M [next slide], thus Kw = [H+] [OH-] = 10-14 M2 Constant ion product! Pure water has equal quantities of H+ and OH- ions, or, put differently, pure water has equal [H+] and [OH-]. [H+] = [OH-] = 10-7 M = 0.1 mM [H2O] Concentration is measured in moles per liter (mol/l) or simply M. 1 l = 1,000 ml of water has a mass of 1,000 g. 1 mole of water has a mass of 18 g (hydrogen 1 Da, oxygen 16 Da). Thus 1 liter of water (1,000 g) contains 1,000 g / 18 g moles of water. [H2O] = (1,000 g / 18 g) M = 55.6 M Base: proton acceptor The pH scale pH = log 1 +] = -log [H [H+] In neutral/pure water [H+] = [OH-] = 10-7 M, so pH = -log(10-7) = -(-7) = 7 Logarithm (base 10) refresher: if log10(x)=y then x=10y Acid: proton donor Strong acids and bases pH = -log [H+] HCl H+ + Cl- HCl is a strong acid that completely dissociates in water. 1 M HCl will thus yield 1 M [H+] and the pH will be pH = -log [H+] = -log(1) = 0 NaOH is a strong base that completely dissociates in water. 1 M NaOH will thus yield 1 M [OH-]. Since [H+] [OH-] = 10-14 M and must remain constant [H+] = 10-14 M and the pH will be pH = -log [H+] = -log(10-14) = 14 Life is compatible only in a narrow pH range around pH 7. Dissociation of a weak acid or weak base O R-C-OH O R-C-O- + H+ (C-term/Asp/Glu) R-NH3+ R-NH2 + H+ (N-term/Lys) HA [H+] [A-] Ka = ––––––––– [HA] A- + H+ (general) = acid dissociation constant Weak acids and weak bases Acetic acid is a weak acid as it does not completely dissociate in water. - A- HA Keq = Ka = + H+ [H+] [A-] [HA] = 1.7x10-5 M [H+] [OH-] Recall for water: K = = 1.8x10-16 M with [H2O] = 55.6 M! eq [H2O] pKa = -log(Ka) = -log(1.7x10-5 M) = 4.8 pKa and pH [A-] [H+] Ka = [HA] when [A-] and [HA] are equal then Ka = [H+]. And thus pKa = 4.8 = -log(Ka) = -log [H+] = pH (since pH is defined as -log [H+]) Titration curves Ka = [H+] [A-] [HA] -] [A pH = pKa + log [HA] +/-1 pH unit Start at low pH and begin to add HO-. The product of [H+] [HO-] must remain constant, so adding HOmeans [H+] must decrease and thus pH increases. At the pKa, [A-] and [HA] are equal, so adding more HOdoes not change the ratio of [A-] to [HA] very much and thus the pH does not change very much (shallow slope of titration curve from ~1 pH unit below pKa to ~1 pH unit above). Measuring pKa values NH4+ - Ka = H+ + NH3 [H+] [NH3] [NH4+ ] pKa = pH when [NH4+] = [NH3] The Henderson-Hasselbalch Equation HA H+ + A- take the -log on both sides Apply definition p(x) = -log(x) Ka = [H+] [A-] [HA] -log Ka = -log pKa = [H+] pH [A-] -log [HA] -] [A -log [HA] and finally solve for pH… -] [A pH = pKa + log [HA] [Proton acceptor] = pKa + log [Proton donor] Acetic acid has a pKa of 4.8. How many ml of 0.1 M acetic acid and 0.1 M sodium acetate are required to prepare 1 liter of 0.1 M buffer with a pH of 5.8? Substitute the values for the pKa and pH into the Henderson-Hasselbalch equation: 5.8 = 4.8 + log [Acetate] [Acetic acid] 1.0 = log [Acetate] [Acetic acid] 10x then *[Acetic acid] on both sides 10 [Acetic acid] = [Acetate] For each volume of acetic acid, 10 volumes of acetate must be added (total of 11 volumes). Acetic acid needed: 1/11 x 1,000 ml = 91 ml Acetate needed: 10/11 x 1,000 ml = 909 ml How does a buffer work? - At the pKa, [HA] = [A-] so the system is able to absorb the addition of HO- or H+. If we add HO- near the pH where [HA] = [A-] (ie pH ~= pKa) then HA can release H+ to offset the HO- added but the ratio of HA to A- does not change much. If we add H+ then A- can absorb H+ to form HA. Hence, the pH does not change much. Buffers are vitally important in biochemical systems since pH needs to be controlled. Living systems must be “buffered” to resist large variations in pH. Phosphate buffering H3PO4 H+ + H2PO4- pKa1 = 2.2 H2PO4- H+ + HPO42- pKa2 = 7.2 HPO42- H+ + PO43- pKa3 = 12.7 Carbonate buffering CO2 + H2O H2CO3 H2CO3 H+ + HCO3- pKa1 = 6.4 HCO3- H+ + CO32- pKa2 = 10.2 Phosphate buffering Carbon dioxide - carbonic acid - bicarbonate buffer CO2 + H2O H2CO3 H2CO3 H+ + HCO3- pKa = 6.4 HCO3- CO3-2 + H+ pKa = 10.2 (not relevant, far from pH 7.4) Carbon dioxide - carbonic acid - bicarbonate buffer If blood pH drops due to metabolic production of H+ then [H2CO3] increases by protonation of HCO3-, H2CO3 rapidly loses water to form CO2(aq), which is expelled as CO2(g). If the blood pH rises, [HCO3-] increases by deprotonation of H2CO3, then breathing rate changes and CO2(g) is converted to CO2(aq) and then to H2CO3 in the capillaries in the lungs. What happens to blood pH when you hyperventilate? What happens to blood pH when you hypoventilate? What is the pH of 0.15 M acetic acid? The pKa of acetate is 4.8, so the Ka = 10-4.8 M = 1.58x10-5 M. O R-C-OH O R-C-O- + H+ [H+] [A-] Ka = _________ [HA] and [H+]=[A-] [HA]=0.15-[H+] [H+]2 [H+]2 Ka = ––––– = ––––––––– = 1.58x10-5 M [HA] 0.15 M - [H+] [H+]2 +1.58x10-5 M [H+] + (-2.37x10-6 M2) = 0 (ax2+bx+c = 0) ax2 + b x + c = 0 Quadratic Formula [H+] = 1.53x10-3 M and thus pH = 2.8 What is the pH of 0.15 M acetic acid? The pKa of acetate is 4.8, so the Ka = 10-4.8 M = 1.58x10-5 M. O R-C-OH O R-C-O- + H+ [H+] [A-] Ka = _________ [HA] and [H+]=[A-] [HA]=0.15-[H+] [H+]2 [H+]2 Ka = ––––– = ––––––––– = 1.58x10-5 M [HA] 0.15 M - [H+] Assumption: [H+] << 0.15 M! [H+]2 = 0.15 M * 1.58x10-5 M [H+]2 = 2.37x10-6 M2 Assumption: [H+] << 0.15 M! [H+] = 1.54x10-3 M or 0.00154 M and thus pH = 2.8 Your 199 prof asks you to make a pH 7 phosphate buffer. You already have 0.1 M KH2PO4. What concentration of K2HPO4 do you need? KH2PO4 H2PO4- + K+ H2PO4- and K2HPO4 HPO42- + H+ [HPO42-] pH = 7 = pKa + log [H2PO4-] 7 = 7.2 + log(x / 0.1 M) -0.2 = log(x / 0.1 M) 10-0.2 = x / 0.1 M x = 0.063 M = [K2HPO4] HPO42- + 2 K+ pKa = 7.2 Phosphate buffering