GRC_BRSH
advertisement

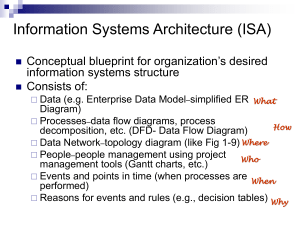
Hydrogen Storage in LiBH4-MgH2-Al Bjarne R. S. Hansena, Dorthe B. Ravnsbæka, Jørgen Skibstedb, Carsten Gundlachc & Torben R. Jensena a Center for Materials Crystallography and Department of Chemistry, University of Aarhus, Langelandsgade 140, DK-8000 Aarhus C, Denmark b Instrument Centre for Solid-State NMR Spectroscopy, Department of Chemistry, and Interdisciplinary Nanoscience Center (iNANO), Aarhus University, Langelandsgade 140, DK-8000 Aarhus C, Denmark c MAX-II laboratory, Lund University, Ole Römers väg 1, 223 63, S-22100 Lund, Sweden Abstract LiBH4 is an interesting hydrogen storage material, as it has a high gravimetric hydrogen content of 18.5 wt% [1]. However, the utilization is hampered by lack of reversibility and high decomposition temperatures. One way of improving metalborohydrides, is the utilization of reactive hydride composites, which has be achieved by adding for instance Al [2] or MgH2 [3]. By adding both MgH2 and Al the LiBH4-MgH2-Al composite further improves the hydrogen release and uptake properties of LiBH4 [4]. In this study the decomposition reactions of LiBH4-MgH2-Al in molar ratios (4:1:1) and (4:1:5) are investigated using in situ Synchrotron Radiation Powder X-ray Diffraction (SR-PXD) and thermal analysis (TGA/DSC) coupled with mass spectroscopy (MS), Sieverts Measurements (PCT), Fourier transform infrared spectroscopy (ATR-FTIR) and solid state 11B Magic Angle Spinning Nuclear Magnetic Resonance (11B MAS NMR) Mechanochemical Synthesis: Fritsch Pulverisette no. 4 with main disk speed: 400 rpm., Relative ratio: -2.25, Mill time: 5 min, Pause time: 2 min, Ball-toPowder mass ratio: 35:1, Repetitions: 24 (Total mill time: 120 min). λ = 1.103671 Å λ = 1.102050 Å Mg/Mg0.9Al0.1 and Al Bragg peak overlap Decomposition reactions (LiBH4-MgH2-Al (4:1:5, S2)) Decomposition reactions (LiBH4-MgH2-Al (4:1:1, S1)) Figures show SR-PXD of LiBH4-MgH2-Al (4:1:1). MgH2 decompose at T = 300 °C, and react with Al to form Mg17Al12 and Mg0.9Al0.1. MgAlB4 is observed at T = 380 °C. This formation might be slow, since diffracted intensity from Mg0.9Al0.1 increase as Mg17Al12 decompose. LiAl is observed at T = 460 °C. From the molar composition LiAl should not form. However, since the formation of MgAlB4 is relatively slow, it is possible that LiAl forms instead since both Al and Li are present. MgH2 decompose at T = 290 °C, but unlike the LiBH4-MgH2-Al (4:1:1) sample Mg17Al12 is not observed. Mg/Mg0.9Al0.1 is however observed after MgH2 decomposition. The formation of MgAlB4 occur at a lower temperature compared to (4:1:1), i.e. T = 300 °C, and seems to form in two different rates. Diffracted intensity from LiAl is observed at T = 410 °C, which is ~50 °C earlier than in (4:1:1). An unknown compound (1) is observed at T = 490 °C and is possibly a stable impurity, as it continues unaltered throughout the experiment. Thermal analysis (comparison) TGA/DSC-MS analysis complement the decomposition pathway observed from in situ data well. No diborane was detected with MS during decomposition of either sample (not shown). At T = 300 °C the decomposition of MgH2 is observed in sample LiBH4-MgH2-Al (4:1:1), but not in the (4:1:5)-sample, although a release of hydrogen is observed in the MS data for both samples. The formation of MgAlB4 is observed as a broad endotherm and the formation of LiAl is seen at T = 410 °C. In LiBH4-MgH2-Al (4:1:1) and LiBH4-MgH2 (2:1) the formation of LiMg is observed. This is however not observed in the in situ SR-PXD measurement (it was only heated to 500 °C) More Al lower Tonset Tonset Tpeak Tend LiBH4-Al (2:1) 390 425 470 LiBH4-Al (2:3) 325 375 450 LiBH4-MgH2 (2:1) 350 420 440 (565) LiBH4-MgH2-Al (4:1:1) 275 415 440 (550) LiBH4-MgH2-Al (4:1:5) 275 350/400 485 Comparison with the LiBH4-Al and LiBH4-MgH2 samples show that the onset temperature for hydrogen release is lowered up to ~75 °C. No B2H6 detected S1: LiBH4-MgH2-Al (4:1:1) S2: LiBH4-MgH2-Al (4:1:5) Temperature (°C) 11B ATR-FTIR MAS NMR S2, LiBH4-MgH2-Al (4:1:5) after 3 PCT cycles Centerband resonance from LiBH4 (-41.3 ppm) Centerband resonance consistent with Li2B12H12 (-9 ppm) * * * * Reversibility PCT desorptions of the LiBH4-MgH2-Al samples show a decrease in hydrogen storage capacity. In S2 (4:1:5) three gas release steps are observed. The release related MgAlB4 is decreasing the most. Hence, the capacity drop is likely related to a boron-containing species. This is also the case in S1 (4:1:1), but LiAl is not observed. LiBH4-MgH2-Al (4:1:1) λ = 1.54056 Å LiBH4-MgH2-Al (4:1:5) λ = 1.54056 Å Diffractograms were obtained after the first desorption and first absorption. These diffractograms confirm the reactions proposed from the PCT desorptions. Unknown 1 is also observed after absorption. Diffracted intensity from LiBH4 is weak compared to the diffractograms of the ball milled samples. LiBH4-MgH2-Al (4:1:1) After 3 PCT cycles λ = 1.54056 Å LiBH4-MgH2-Al (4:1:5) After 3 PCT cycles λ = 1.54056 Å * * * * * * * * * = spinning sidebands/satellite transitions The 11B MAS NMR spectra of S2 after three hydrogen release and uptake cycles show a centerband resonance from LiBH4, and a chemical shift consistent with Li2B12H12 which was not detected by PXD. This is also the case in S1. Li2B12H12 is a stable closo-borate and it does not seem to react/rehydrogenate at the physical conditions used in this study. Li2B12H12 was also observed by FT-IR in the cycled samples. Hence, Li2B12H12 is likely responsible for the hydrogen storage capacity loss in the samples. More information: brsh@chem.au.dk Acknowledgements and references Sincere acknowledgements are directed to iNANO, Danscatt , MAX-Lab Bor4Store (EU) and Center for Materials Crystallography (CMC) [1] L. Schlapbach, Nature 2009, 460, 809-811; [2] D. B. Ravnsbæk, T. R. Jensen, J. Appl. Phys. 2012, 111, 112621 ; [3] U. Bösenberg, S. Doppiu, L. Mosegaard, G. Barkhordarian, N. Eigen, A. Borgschulte, T. R. Jensen, Y. Cerenius, O. Gutfleisch, T. Klassen, M. Dornheim, Acta Materialia 2007, 55, 3951–3958; [4] Y. Zhang, Q. Tian, H. Chu, J. Zhang, L. Sun, J. Sun, Z. Wen, J. Phys. Chem. C 2009, 113, 21964–21969