Ratiometric fluorescent probes for sensing interactions of peptides
advertisement
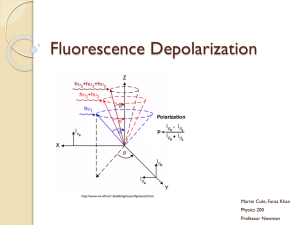
Ratiometric fluorescent probes for sensing interactions of peptides with their molecular targets Viktoriia Postupalenko, Andrey Klymchenko, Oleksandr Stryzhak ,Vasyl Pivovarenko, Yves Mély 1. Laboratoire de Biophotonique et Pharmacologie, UMR-CNRS 7213, Faculté de Pharmacie, Université de Strasbourg, Illkirch, France 2. Department of Chemistry, Kyiv National Taras Shevchenko University, Ukraine Proteins DNA/RNA Proteins Membranes Proteins Fluorescence is universal method to report protein interactions with different targets Environment-sensitive probes _ + nonpolar O h h’ N Prodan _ + Polar Poor solvation N O Water: Fluorescence Intensity Oil: Polarity 4 3 2 1 0 500 550 600 Wavelength, nm 650 Environment-sensitive probes change their color with the change of polarity Two color probes: principles Tautomeric Т* Normal N* O O O O H O H Т* emission N* emission h Т* CH3CN Polarity ESIPT O N* N N CH2Cl2 EtOAc N N O O O O H Toluene 500 600 (nm) O O H Excited state proton transfer (ESIPT) results in two emission bands Spectra highly depend on environment properties Protein – nucleic acid interactions Spectroscopic properties of the 3HC label Buffer MeOH EtOH Acetonitrile Toluene 1.5 1.2 0.9 Polarity Fluorescence Intensity, a.u. 1.8 T* N* O H N HO 0.6 O 3HC O OH O O 0.3 0.0 400 450 500 550 600 650 Wavelength, nm Shvadchak et al. Nucleic Acids Res. 2009 N*/T* band ratio strongly increases with polarity and H-donor ability Hydration shifts the T* band position to the blue Peptide-nucleic acid interactions Free peptide O Fluorescence Intensity, a.u. HO 1.0 O H N O C N F O C KNVK 11 O G K EG H Zn G K EGH C Q K M Zn 37 W K D C C C RAPRKKG TERQAN 55 T A R N 3HC-NC 0.8 h 0.6 0.4 Peptide + SL3 RNA Proximity sensing 0.2 0.0 400 450 500 550 600 650 DNA Wavelength, nm Shvadchak et al. Nucleic Acids Res. 2009 N*/T* ratio decreases after peptide-nucleic acid interaction Fluorescent amino acid analog H COOH HO O NH2 H N COOH O NH2 NH2 O COOH O 3HC-L-amino acid L-Tryptophane NC mutants with 3HC-amino acid Ala Phe Trp All NC mutants preserve original peptide activity Interaction with nucleic acids Complex with SL3 RNA Fluorescence Intensity, a.u. 1.0 0.8 0.6 Ala Free Ala peptide Phe Trp Ala Trp 0.4 0.2 Phe 0.0 400 450 500 550 600 650 Guzman et al. Science, 1998 Wavelength, nm Probe response correlates with 3D structures of peptide/nucleic acid complex Protein – membrane interactions Spectroscopic properties of the MFL label O H N HO O O OH O Fluorescence intensity, a.u. MFL 1.0 0.8 0.6 N Toluene EtOAc Acetone CH3CN 2 6 21 36 H2O 80 Model peptides: Melittin Magainin-2 0.4 0.2 Polylysine (PLL) 0.0 450 500 550 600 Wavelength, nm 650 700 Protic environment – one-band fluorescence Aprotic – two emission bands + + + + + + Fluorescence Intensity, a.u. Binding of the peptides to lipid membrane 10 8 Melittin bound to vesicles (DOPC) 6 4 LUV models for the cellular membrane 2 Free peptide 0 450 500 550 600 650 700 Wavelength, nm Free peptide is poorly fluorescent – one emission band Bound to liposomes – dual emission DOPC:DOPS (8:2) = 38 1.0 Magainin-2 Melittin Polylysine 0.8 = 10 0.6 0.4 F (with quencher) / Fo (without quencher) Fluorescence Intensity, a.u. Analysis of N-terminus insertion into membranes surface medium deep 0.8 16.5 Å 0.7 8Å 0.6 8Å 0.5 0.4 Magainin 2 Melittin H2O, = 80 0.2 N O O P O O O 0.0 O O 500 550 600 650 700 Ratio of the two emission bands of the probe correlates with the depth of insertion 5.85 Å Wavelength, nm 12.15 Å 450 = 2-3 19.5 Å O Poly-L-lysine Localization of N-terminus of peptides in the membrane N O O O N O P O O O O O P O O O P O N + O + + + + O O O O O O O N O O 16.5 Å Polylysine 8Å Melittin & Magainin-2 O O P O O O O + O O O NC – vesicles interaction O HO O H N O G C N F O C MQRGNFRNQRKNVK 1 N KEGH Zn EG H GK T Q C A M K R K Zn N W D C C TERQAN RAPRKKG C 55 MFL-NC Free peptide DOPC (0) DOPS (-1) DOPG (-1) 8 6 4 2 0 Magainin Melittin Polylysine NCp7(1-55) 1.2 1.0 Fluorescence, a.u. Fluorescence Intensity, a.u. -1 0.8 0.6 0.4 0.2 0 0.0 450 500 550 600 Wavelength, nm 650 700 450 500 550 600 650 700 Wavelength, nm NC interacts with negatively charged vesicles but only marginally with neutral ones The N-terminus of peptide locates 17 Å from the center of bilayer Conclusions Environment-sensitive 3HC probe can be monitoring peptide-nucleic acids interactions. used for Proposed fluorescent amino acid analog reports binding of NCp7 to oligonucleotides and site-selectively monitors the environment in NCp7 complexes. MFL label reports binding to membrane by appearance of two emission bands and increase in quantum yield. Ratio of the two emission bands of the probe correlates with the insertion depth (from parallax quenching). Thank’s for your attention!