Chemistry Courses: About
advertisement
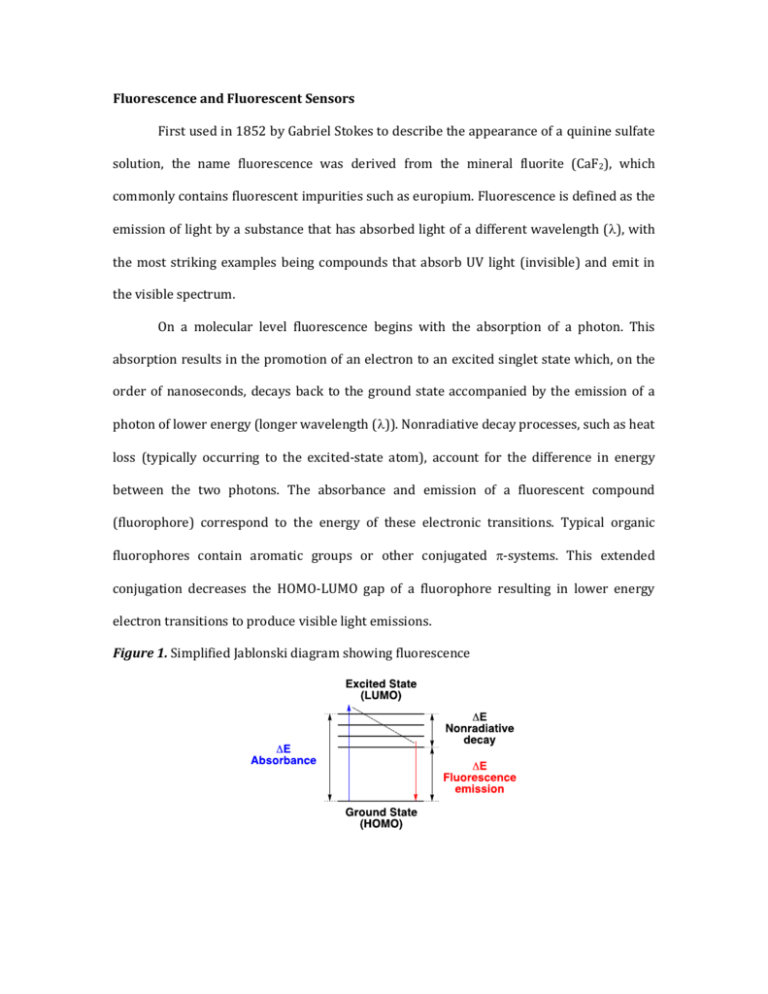
Fluorescence and Fluorescent Sensors First used in 1852 by Gabriel Stokes to describe the appearance of a quinine sulfate solution, the name fluorescence was derived from the mineral fluorite (CaF2), which commonly contains fluorescent impurities such as europium. Fluorescence is defined as the emission of light by a substance that has absorbed light of a different wavelength (), with the most striking examples being compounds that absorb UV light (invisible) and emit in the visible spectrum. On a molecular level fluorescence begins with the absorption of a photon. This absorption results in the promotion of an electron to an excited singlet state which, on the order of nanoseconds, decays back to the ground state accompanied by the emission of a photon of lower energy (longer wavelength ()). Nonradiative decay processes, such as heat loss (typically occurring to the excited-state atom), account for the difference in energy between the two photons. The absorbance and emission of a fluorescent compound (fluorophore) correspond to the energy of these electronic transitions. Typical organic fluorophores contain aromatic groups or other conjugated -systems. This extended conjugation decreases the HOMO-LUMO gap of a fluorophore resulting in lower energy electron transitions to produce visible light emissions. Figure 1. Simplified Jablonski diagram showing fluorescence Figure 2. Common structural motifs in organic fluorophores Fluorescence is a useful property with practical applications in analytical chemistry, biology, earth sciences and electronics (among others). One notable application of this property is in the development of fluorescent chemosensors: abiotic molecular devices whose fluorescent properties can respond to external stimuli in a quantifiable manner. The ability of these materials to function on the molecular level provides numerous advantages over classical techniques, for example chemosensors allow for the in vivo quantification of metals involved in cellular signaling. Fluorescence offers desirable attributes for sensing applications, such as high sensitivity of detection down to the single molecule, “on-off” switchability, subnanometer spatial resolution, submicron visualization and submillisecond temporal resolution. Quantitative data can readily be obtained for specific targets by operationally simple detection schemes exploiting binding-induced perturbations of deexcitation pathways such as photoinduced electron transfer (PET) or intramolecular charge transfer (ICT). It should be noted that methods such as internal charge transfer (ICT) and photoinduced electron transfer (PET) typically result in probes that exhibit either ratiometric or “turn-on” responses, allowing sensitive analyses capable of detecting and quantifying extremely small quantities of a given analyte. Changes in the intensity and/or wavelength of light emitted from molecular probes have been used in the detection of heavy metal ions and anionic metabolites; in the labeling of amino acids, peptides, nucleotides, and other macromolecules; and for monitoring reactive oxygen/nitrogen species. Common fluorescence terminology and concepts: Stokes fluorescence is when the fluorescence emission is of a lower energy (longer ) than the light absorbed (em > abs and Eabs > Eem). This is the most common type of fluorescence. Nonradiative decay pathways such as heat loss account for the energy differences. Anti-Stokes fluorescence is when the fluorescence emission is of a higher energy (shorter ) than the light absorbed (em < abs and Eabs < Eem). Stokes shift describes the difference between the emission wavelength (em) and the absorbance wavelength (abs). Molar absorbance () is the parameter describing the ability of a molecule to absorb light at a particular wavelength. The absorbance E measured at any wavelength on a spectrophotometer is proportional to the concentration c (in mol/l) and path length in a sample l (in cm). This relationship is represented by the Beer-Lambert law equation E = cl. Fluorescence quantum yield (φF) is defined as photons emitted over photons absorbed. While the maximum fluorescence quantum yield (φF) is 1 for any given compound, this value is never observed in solution, and compounds with quantum yields as low as 0.1 are still considered highly fluorescent. Bathochromic shift is the change in a spectral band to a longer wavelength (). This term is informally called a red shift, however it has no relation to the Doppler shift. Examples of how the term is used include; spectral changes in substituted series of molecules and spectral changes due to environmental changes such as polarity (i.e. solvatochromism). Hypsochromic shift is the change in a spectral band to a shorter wavelength (). This term is informally called a blue shift, however it has no relation to the Doppler shift. Examples of how the term is used include; spectral changes in substituted series of molecules and spectral changes due to environmental changes such as polarity (i.e. solvatochromism). Photobleaching is the photochemical destruction of a dye or fluorophore. “Turn-on” fluorescent sensors respond to analytes with an increase in fluorescence intensity. These sensors commonly utilize a PET mechanism. Ratiometric fluorescent sensors respond to analytes with changes in the relative intensities of two emission bands. These sensors, which commonly utilize an ICT mechanism, allow for obtaining internally self-calibrated response signals. Photoinduced Electron Transfer (PET) Photoinduced electron transfer (PET) a commonly employed de-excitation pathway for development of “turn-on” fluorescence chemosensors. PET systems are composed of three components, a fluorophore, a linker and a receptor. The receptor component typically possesses a non-bonding electron pair, typically of a nitrogen atom, whose energy lies between the HOMO/LUMO gap of the fluorophore. Electron transfer from the non-bonding lone pair of the receptor to the excited state fluorophore quenches fluorescence. For PET to occur, the energy of the fluorophores excited state must be sufficient to oxidize the receptor and reduce the fluorophore. Binding of a cation to the receptor lone pair raises the oxidation potential, making PET thermodynamically disfavored and restoring fluorescence to the system. This attribute makes PET systems ideal candidates for the detection of metal ions as their “turn-on” mechanism is activated by coordination events. Attributes of PET Sensors: Receptor and fluorophores are closely linked but separate electronic systems Relatively insensitive to polarity of local environment Non-bonding receptor electrons participate in redox chemistry with the fluorophores quenching fluorescence. Protonation or coordination lowers the energy of receptor electrons, inhibiting the PET process and restoring fluorescence. Most general mechanism for fluorescent sensing Figure 3. Spectral and visual example of a PET sensor (left) and simplified orbital depiction of the PET process (right) *For ease of communication HOMO and LUMO were used. These terms only apply to the ground state. The figure actually depicts singly occupied molecular orbitals (SOMO). Intramolecular Charge Transfer (ICT) Intramolecular charge transfer (ICT) is a common mechanism utilized in ratiometric fluorescent chemosensors. ICT and PET compounds are structurally distinct from one another, with ICT compounds including the receptor and fluorophore in the same electronic system. Correspondingly, ICT systems are characterized as “charge-polarized” states, as compared to the “charge-separated” states of PET systems. The two sensing mechanisms are easily differentiated spectroscopically. ICT states (or charge transfer (CT) states) are fluorescent, as compared to quenched PET states. Typically the CT states show highly shifted emission bands. Upon binding analyte the CT states become inaccessible and the normal fluorescence or locally excited (LE) state is restored, resulting in two wavelength emissions. Compounds displaying ICT are highly polarized, possessing electron rich and electron poor regions or functional groups in their conjugated -systems. In the excited state electron donor groups become more donating and electron withdrawing groups more withdrawing, which results in a greater excited state molecular dipole. In polar solvents the dipole is stabilized, resulting in a lower energy CT state and a bathochromic shift in emissions. ICT sensing can be achieved by binding analytes to either the donor or acceptor region of the fluorophore. A common example is binding a cation at an electron-donating dialkylamino group. Binding the nitrogen lone pairs removes their electron donating ability, making the long wavelength CT state inaccessible and restoring the shorter wavelength LE state. Attributes of ICT Sensors: Receptor and fluorophores are part of the same electronic system Often sensitive to the polarity of local environments Polarized molecules containing electron donor and acceptor components Interactions with analytes influence the polarization of the fluorophore, which can alter emission intensity and the wavelength of the absorption and emission. Analyte induced shifts in emission wavelength (em) allow for ratiometric analysis, providing sensors with their own internal standard. Figure 4. Spectral and visual example of an ICT sensor (left) and simplified orbital depiction of the ICT process (right) Please see Introduction to Fluorescence Sensing by Alexander P. Demchenko (especially chapters 1 and 6) for more background information.