Chap. 8 Post-transcriptional Gene Control
Topics
• Processing of Eukaryotic Pre-mRNA
• Regulation of Pre-mRNA Processing
• Cytoplasmic Mechanisms of Post-transcriptional Control
Goals
• Learn the mechanisms of 5' capping
and polyadenylation.
• Learn the mechanism of pre-mRNA
splicing by the spliceosome complex.
• Learn the general functions of
splicing repressors and activators in
regulation of pre-mRNA splicing.
• Learn about mechanisms for
translation control via targeted
RNA degradation.
hnRNP-stained lampbrush chromosome
Post-transcriptional
Gene Control
Post-transcriptional gene
control refers to all of the
processes that regulate gene
expression subsequent to
transcription initiation (Fig.
8.1). These processes include
regulation of alternative
splicing, RNA editing, and
RNA degradation. With the
exception of alternative
splicing, these mechanisms
typically are involved in the
regulation of only a relatively
small fraction of RNAs in a
cell. However, they can be
highly important for
regulation of a given gene.
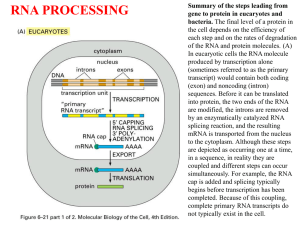
Overview of Pre-mRNA Processing
Pre-mRNA processing includes 5' capping, 3’ polyadenylation, and
intron splicing (Fig. 8.2). These reactions occur in the nucleus,
and begin while the primary transcript is being elongated (cotranscriptional). Mature mRNAs then are transported to the
cytoplasm for translation.
Eukaryotic pre-mRNA Processing: Capping
Bacterial mRNAs are functionally active
as transcribed. Eukaryotic pre-mRNAs
must be extensively processed to attain
their final functional forms. The
modification that occurs at the 5' end
of the primary transcript is called the
5' cap (m7Gppp) (Fig. 4.14). In this
modification, a 7-methylguanylate
residue is attached to the first
nucleotide of the pre-mRNA by a 5'-5'
linkage. The 2'-hydroxyl groups of the
ribose residues of the first 2
nucleotides may also be methylated. The
5' cap is important for transport of the
mRNA to the cytoplasm, protection
against nuclease degradation, and
initiation of translation.
Mechanism of 5' Capping
The synthesis and structure of the
5' cap that is added to most
vertebrate mRNAs is illustrated in
Fig. 8.3. Caps are added to
mRNAs and snRNAs transcribed by
RNA Pol II. Capping enzyme
removes the phosphate from the
5’ end of the pre-mRNA and adds
the 5'-5'-linked guanylate residue
to the end of the RNA. Capping
enzyme associates with RNA Pol II
via its phosphorylated CTD. Other
enzymes add the methyl groups to
N7 of the 5' guanylate and to 2'hydroxyl groups of the first one or
two nucleotides in the primary
transcript. (S-Ado-Met: Sadenosylmethionine).
Heterogeneous Ribonucleoprotein Particles
Pre-mRNAs and other nuclear RNAs are collectively known as
heterogeneous nuclear RNA (hnRNA). hnRNA is extensively
bound to binding proteins, and complexes between hnRNA and
protein are called heterogeneous ribonucleoprotein particles
(hnRNP). Binding proteins function by preventing hnRNA from
forming tangled 2˚ structures that would otherwise interfere
with processing reactions. As illustrated in Fig. 8.5, many RNAbinding proteins contain an RNA recognition motif (RRM) that
binds RNA via positively charged amino acids. The cover figure
for Chap. 8 shows the extensive hnRNP content of highly
transcribed lampbrush chromosomes in newt oocytes.
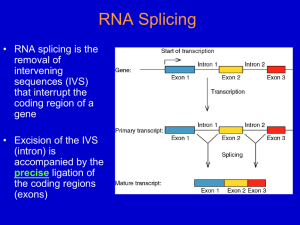
Intro to pre-mRNA Splicing
In higher eukaryotes, nearly all genes contain intron sequences
that must be spliced out of pre-mRNA to form mature mRNA
species. One of the earliest (1977) experiments showing that
introns are present in genes is shown in Fig. 8.6. In this
experiment, a double-stranded DNA fragment containing most of
the adenovirus hexon gene was denatured, hybridized with the
hexon mRNA, and then viewed under the electron microscope. As
shown in the micrograph and the schematic diagram on the right,
DNA loop sequences corresponding to introns removed from the
mRNA can be seen looping out from the DNA/RNA hybrid.
Splice Site Consensus Sequences
Pre-mRNA splice site consensus sequences located at the extreme
ends of introns help direct splicing reactions (Fig. 8.7). The
identities of these sequences were learned by comparing the
sequences of genes to their spliced mRNA products. The GU
dinucleotide at the 5' splice site of the intron and the AG
dinucleotide at the 3' splice site are highly conserved. Also highly
conserved within the intron is a branch point sequence containing
the branch-point A residue located ~20-50 nucleotides upstream
of the 3' splice site. The remaining central region of the intron
(not shown) generally is unimportant for splicing.
Mechanism of the Splicing Reaction
The splicing reaction occurs via
2 transesterification reactions,
for which ∆Gsum ~ 0 (Fig. 8.8).
Thus no energy input is required
for splicing. In the first
reaction, the free 2'-hydroxy
group of the branch point A
residues attacks and cleaves
the phosphodiester linkage at
the 5' splice site. In the
second reaction, the 3'hydroxy group of the 5' exon
attacks and cleaves the
phosphodiester linkage at the
3' splice site. The products of
the second reaction are the
spliced mRNA product and the
excised intron, which is called
the lariat product. The lariat
intron RNA is degraded.
Small Nuclear RNAs (snRNAs) and Splicing
The splicing reaction requires 5 snRNAs (U1, U2, U4, U5, &
U6) that range from about 100-200 nucleotides in length. Each
snRNA forms a complex with 6-10 proteins which are called
small nuclear ribonucleoprotein particles (snRNPs, pronounced
"snurps"). snRNAs bind to pre-mRNA and each other within a
larger splicing complex known as the spliceosome (next slide).
Interactions between the U1 snRNA and the 5' splice site, and
the U2 snRNA and the branch point sequence are crucial in
selecting where splicing occurs (Fig. 8.9a). Note that the
branch point A residue bulges out of the U2-pre-mRNA duplex.
Sm sites indicate where snRNP proteins bind to the snRNAs.
Spliceosome Reactions (I)
Spliceosomes are large supramolecular
complexes consisting of 5 snRNPs and the
pre-mRNA. The assembly of the
spliceosome and splicing reactions begin
with a complex between the pre-mRNA
intron, the U1 snRNP bound to the 5'
splice site, and the splicing factors SF1
and U2AF bound to the branch point A
and pyrimidine tract/3’ AG of the intron,
respectively (Fig. 8.11, top ). In Step 1,
SF1 departs and the U2 snRNP adds to
the complex. In Step 2, the U4/U6/U5
complex adds on forming the fully
assembled spliceosome. In Step 3, the
U1 and U4 snRNPs depart, and the premRNA is repositioned in the complex for
splicing. (Continued on the next slide.)
Spliceosome Reactions (II)
The transesterification reactions
occur in Steps 4 & 5 via the
mechanism shown in Fig. 8.11.
Following splicing, the remaining
components of the complex
disassemble. In Step 6, a nuclease
known as debranching enzyme
cleaves the 2'-5' branch point
linkage in the lariat. Degradation
of the lariat to individual
nucleotides by 3'-to-5'
exonucleases then ensues (not
shown). It is estimated that ~95%
of the polymerized nucleotides
within pre-mRNAs ultimately are
degraded back to single nucleotides
following splicing.
RNA Pol II CTD Binds Pre-mRNA
Processing Factors
Enzymes involved in 5' capping, polyadenylation, and splicing
bind to the long phosphorylated CTD of RNA Pol II (Fig. 8.12)
while it is transcribing a gene. This ensures that these factors
are delivered to the pre-mRNA sites where they are needed.
Current research indicates that the binding of these factors to
phosphorylated CTD is required to ensure that the enzyme
remains processive. Thus, transcription will occur only if these
factors are present in sufficient supply.
Exon Recognition in Long Pre-mRNAs
The average human intron is ~3,500 nucleotides in length, while
the average exon is only ~150 nucleotides long. The longest
introns are 500 kb in length. As shown in Fig. 8.7, splice site
consensus sequences are fairly degenerate, and in long introns,
multiple potential 3' acceptor sites occur. Remarkably, exon
sequences play an important role in splice site selection in many
long introns (Fig. 8.13). Exons contain exonic splicing enhancers
(ESEs) that bind SR proteins which recruit the U2 snRNP &
U2AF factor to 3' splice sites, and the U1 snRNP to 5' splice
sites flanking exons. These assemblies are known as cross-exon
recognition complexes. Through this mechanism, the correct
splice junctions within a long pre-mRNA are accurately selected.
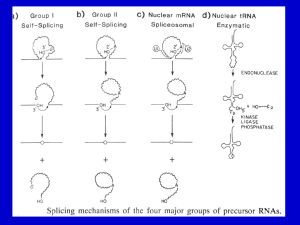
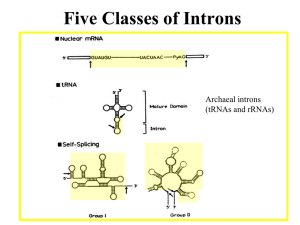
Self-splicing Introns
Introns in some protozoan rRNA primary transcripts (group I
introns) are self-splicing. Likewise, introns in some protein, rRNA,
and tRNA transcripts produced from mitochondrial and chloroplast
genes in plants and fungi (group II introns) also carry out selfsplicing reactions. The study of intron self-splicing lead to the
discovery of catalytic RNA (ribozymes). Self-splicing introns have
strongly conserved secondary and tertiary structures. Because the
structure of snRNAs in the spliceosome complex resembles that of
group II introns (Fig. 8.14), it is speculated that the spliceosome
machinery evolved from group II introns. Early in evolutionary
history, when catalytic RNAs may have been much more prevalent,
all introns may have been excised by self-splicing. The transfer of
splicing reactions to snRNA would have removed constraints on the
structure of introns, and thereby facilitated exon shuffling and
gene evolution.
3' Cleavage and Polyadenylation of PremRNAs (I)
3' cleavage and polyadenylation of
mRNAs are tightly coupled
processes that are signaled by 2
sequences near the 3' end of premRNA. These sequences serve as
binding sites for 4 nuclear factors
(Fig. 8.15). In Step 1, CPSF
(cleavage and polyadenylation
specificity factor), CStF (cleavage
stimulatory factor), and CFI/II
(cleavage factors I & II) bind to
these sites. Then in Step 2, PAP
(poly(A) polymerase) binds to the
complex. In Step 3, the premRNA is cleaved just downstream
of the AAUAAA poly(A) signal.
(Continues on the next slide)
3' Cleavage and Polyadenylation of PremRNAs (II)
In Step 4, the CStF, CFI, and
CFII factors and the 3'
fragment from the pre-mRNA
are released. The RNA fragment
is rapidly degraded. PAP then
begins slow polymerization of the
poly(A) tail. In Step 5, PABII
(PABPII, poly(A)-binding protein
II) adds to the complex and
stimulates rapid polymerization of
the remainder of the poly(A) tail
(Step 6). PABPII also controls
the length of the poly(A) tail
which typically ranges from 200250 residues. PABPII binds the
RNA via a RRM binding sequence.
As discussed in Chap. 4, the
poly(A) tail functions in
translation and mRNA turnover.
Intro to Control of Alternative Splicing
The most common mechanism by which post-transcriptional gene
control is achieved is the regulation of alternative splicing. In
humans, ~95% of genes are specified by complex transcription
units that produce different protein isoforms due to alternative
splicing. Alternative splicing is very common in the nervous
system. Alternative splicing is regulated by splicing repressors
and activators that control splice site selection.
Regulated Splicing in Drosophila Sexual
Differentiation (I)
One of the best understood systems where alternative splicing is
used to regulate gene expression is that used in the control of
sexual differentiation in Drosophila embryos. Sexual
differentiation is controlled by the sex-lethal (sxl), transformer
(tra), and double-sex (dsx) genes (Fig. 8.16). Sxl is a female
specific splicing repressor that is not synthesized in males. Sxl
not only regulates the splicing of its own primary transcript
but also regulates
splicing of the premRNA encoding the
Tra protein in
females. The Sxl
and Tra isoforms
produced in males
are non-functional
due to the presence
of stop codons in
exons 3 and 2 of
these respective
genes. These exons
are skipped in
alternative splicing
of the sxl and tra
transcripts in
females.
Regulated Splicing in Drosophila Sexual
Differentiation (II)
Tra protein is a splicing activator. Its expression in females
results in the synthesis of the female isoform of Dsx. Its absence
in males, results in the synthesis of the male isoform of Dsx. The
female form of Dsx is a transcriptional repressor of male
differentiation genes. The male form of Dsx is a transcriptional
repressor of female differentiation genes. Thus alternative
splicing of the sxl gene ultimately determines sex.
Mechanism of Action of Tra Protein
The Tra splicing activator regulates splice site selection in female
embryos by binding to a complex between the Rbp1/Tra2 SR
proteins bound to exonic splicing enhancer sequences in the 4th
exon of the dsx primary transcript (Fig. 8.17). Binding directs
the assembly of the U2 snRNP and the U2AF protein at the 3'
end of the intron preceding exon 4. Thus, the 4th exon is spliced
into the dsx mRNA in females. This exon is skipped over in
splicing of the male dsx transcript. The protein domain encoded by
the 4th exon is important in determining the repressor activity of
the Dsx TF.
Gene Repression by miRNA & siRNA
Two post-transcriptional mechanisms for inhibition of gene
expression by small single-stranded RNAs were discovered
relatively recently in C. elegans. Micro RNAs (miRNAs) inhibit
gene expression by blocking the translation of complementary
mRNAs. Humans express about 500 miRNAs, and some plants
express over 106 miRNAs. Because a single miRNA can bind to
more than one target mRNA, it is estimated that about 1/3
of all human genes may be regulated by miRNAs. Short
interfering RNAs (siRNAs) inhibit gene expression by
specifically targeting a complementary mRNA for degradation.
The mechanism of gene silencing by siRNA is known as RNA
interference (RNAi) and is an important research tool. RNAi
is thought to play a natural role in protection of cells from
RNA viruses and retrotransposons.
Structures of miRNA & siRNA
Both miRNAs and siRNAs are 21-23-nucleotide single-stranded
RNAs. miRNAs bind to the 3' UTR regions of complementary
mRNAs via imperfect base-pairing (Fig. 8.25a). Thus they
often can inhibit translation of more than one mRNA. siRNAs
hybridize perfectly without any mismatches to the coding region
of their target mRNAs (Fig. 8.25b). Thus they typically
regulate only a single mRNA species.
Mechanism of Action of mi- and siRNAs
miRNAs are produced by the
mechanism shown in Fig. 8.26. RNA Pol
II transcribes pri-miRNA transcripts
that are partially double-helical. The
pri-miRNA is processed to a shorter ~
70 nt pre-miRNA that is then
transported to the cytoplasm. The
pre-miRNA, which folds into a hairpin
structure, is bound by a protein
complex containing the enzyme known
as Dicer. Dicer cleaves the molecule
producing a 21-23-nt double stranded
miRNA. Finally, one of the strands is
bound by a protein complex known as
the RISC complex (RNA-induced
silencing complex). The RISC/miRNA
complex subsequently binds to the 3’
UTR of a target mRNA leading to its
sequestration away from ribosomes.
siRNAs are generated in the Dicer
reaction from double-helical RNAs
introduced into cells or produced from
cleavage of viral RNAs. They also are
bound by the RISC complex. However,
RISC/siRNA complexes bind to the coding region of a target
mRNA, and ultimately the target mRNA is cleaved at a site
within the perfect siRNA-mRNA duplex (Fig. 8.25b, arrow).
RNA Interference (RNAi)
In RNA interference, short
interfering RNAs (siRNAs, ~21
nts) produced from longer dsRNAs
specifically block gene expression
by binding to a target mRNA and
triggering its degradation.
dsRNAs can be transcribed in
vitro and injected into an embryo,
for example, where processing by
the enzyme known as dicer
produces the siRNA (Fig. 5.45 a
& b). Alternatively, dsRNA can be
expressed in vivo in response to
some signal. Subsequent
processing to siRNA by dicer then
triggers mRNA degradation (Fig.
5.45c). RNAi-mediated gene
inactivation is commonly applied to
silence gene expression in C.
elegans, Drosophila, plants, and
even mice. The mechanism by
which siRNAs cause mRNA
degradation is covered in Chap. 8.