Lec. 19
advertisement

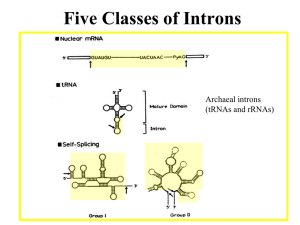
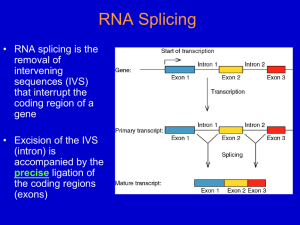
Consensus Group II intron Structure 6 domains, “Helical Wheel” Domain I contains binding sites for the 5’ exon (keeps the 5’ exon from floating away after the first splicing step) Group II Splicing Pathway (1) The 2’ OH of a special internal A attacks the 5’ splice site creating a branched intron structure. (2) The 3’ OH of the 5’ exon attacks the 3’ splice site, ligating the exons and releasing the intron as a lariat structure. Structure of NmRNA Introns 1. Most begin with GU and end with AG. 2. Most of the internal sequences are not conserved. 3. However, there are other important consensus sequences near the ends (in addition to GU and AG). Consensus Mammalian NmRNA Splice Signals 5’ ag/GUAAGU -------YNCURAC---YnNAG/g 3’ Y - pyrimidine (U or C) Yn - string of ~ 9 pyrimidines R - purine (A or G) N - any base Branch site sequence Nm RNA Splicing Mechanism: How it has been studied. • Elucidating the overall mechanism, cis elements, and trans factors depended on: 1. Site-directed mutagenesis of genes in vitro, and subsequent expression in vivo (yeast, Hela cells, and others). 2. Development of accurate splicing extracts (HeLa cells and yeast). 3. Isolation of temperature-sensitive yeast mutants defective in NmRNA splicing. In yeast, the branch-point sequence determines which downstream AG is used. Exon 1 branch Exon 2 Inserted sequence Branch sequence Branch site moved into exon 2. RNAs that were tested for splicing in vivo. Fig. 14.8 Exon 2 Kinetics of In vitro Splicing in a Hela cell nuclear extract Pre - radioactively labeled precursor RNA - The splicing reactions were separated by gel electrophoresis. Notice that the intron and intron-exon RNAs have an unusually reduced mobility in these polyacrylamideurea gels. There is also some cleavage at the Exon 2 - Intron 2 splice-site, producing the Spliced Exons molecule. Spliced exons Fig. 14.5 Plot of changes in amounts of products and intermediates during the splicing reaction in the previous slide. Fig. 14.5 NmRNA Splicing occurs on Spliceosomes! Strategy: An Adenovirus pre-mRNA (32Plabeled) was incubated in a HeLa cell nuclear extract to allow splicing to begin. Then the extract was centrifuged down a glycerol gradient to size the complexes that formed. Result: The intron-exon 2 intermediate sedimented at ~60S on a glycerol gradient – much bigger than expected for naked RNA. Question: What else is in the 60S “spliceosome” complex? Fig. 14.13, 2nd ed. Spliceosomes contain Snurps (snRNPs, small nuclear ribonucleoproteins) 1. A snurp contains a small, nuclear, U-rich RNA (snRNAs = U1, U2, U4, U5 or U6), and > 7 proteins, 7 (Sm) are common. 2. The snRNAs base-pair with the pre-mRNA (U1, U2, U5, U6) and/or with each other (U4-U6 and U2-U6). 3. Lupus patients have antibodies to snurps; mainly the Sm proteins. Fig. 14.28 Structure (in stereo) of the U1 SnRNP Proteins 70K, A, and C are specific to U1 snurp U1 and U2 paired with pre-mRNA in yeast Roles of snRNAs/Snurps 1. U1 pairs with the 5’ splice-site. 2. U2 pairs with the branch point; also pairs with U6 in the assembled spliceosome. 3. U4 pairs with U6 in SnRNPs, but unpairs during spliceosome assembly. 4. U5 interacts with both exons (only 1-2 nt adjacent to intron); helps bring exons together. 5. U6 displaces U1 at the 5’ splice-site (pairs with nt in the intron); it also pairs with U2 in the catalytic center of the spliceosome. Similar active sites (catalytic center) in Spliceosomal and Group II introns? (both models after first step) Fig. 14.22 The Spliceosome Cycle of Assembly, Rxn, and Disassembly Fig. 14.27 Intermediate complexes in the Spliceosome cycle • CC is the commitment complex (contains U1 on the pre-mRNA) • A also contains U2 • B1 also contains U6-U4/U5 • B2 lacks U1 and U4, “activated spliceosome” • C1 contains 5’-exon & intron-exon • C2 contains intron-lariat and ligated exons Some Unique Features of the Spliceosome 1. Transient complex that forms on pre-mRNA. Contrast with ribosomal subunits, which are completely stable. 2. Ribonucleoprotein components of the spliceosome, snurps, are stable structures. 3. In yeast, the spliceosome sediments at ~40S whereas in humans it is ~60S (ribosomal subunits from these species are similar in size). Proteins that promote formation of the Commitment Complex • In humans: the SR proteins SC35 and SF2 commit splicing on globin & HIV Tat premRNA – SR proteins have domains rich in serine and arginine • In yeast: the branch-point bridging protein (BBP) binds to the U1 snurp at the 5’ end of the intron, and the RNA and Mud2p protein near the 3’ end of the intron – Helps define the intron prior to splicing Fig. 14.34a Figure 14.36 SS - splice site BP - branch point Branch-point Bridging Protein (BBP) binds RNA (near the 3’ end of intron) and 2 proteins (U1 SnRNP & Mud2p). Helps define the intron portion.