Explain concentration gradient and diffusion with a picture or diagram..
advertisement
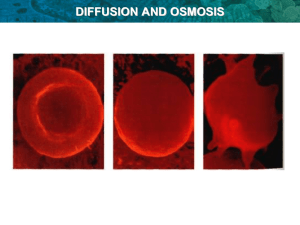
Bellwork: Staple Current Events: • • • • Rubric Written Analysis Article MLA Cite Reference 1. Explain concentration gradient and diffusion with a picture or diagram.. Also write a real-life example of diffusion Concentration Gradient and Diffusion Team 1 Leah, David, Nick Concentration Gradient The difference of a concentration of solutes in a single solution between two regions. Diffusion The process by which molecules move from an area of high concentration to low concentration http://highered.mcgrawhill.com/sites/0072495855/student_view0/chapter2/animation__how_diffusion_w orks.html Sugar dissolving in water. Sugar has a higher concentration than the water around it. The particles will move randomly until they are evenly distributed among the water. This is more commonly known as dissolving the sugar, but the process is called diffusion. Example Molecules Solution Lid Increase of Temperature or Pressure Heat The difference from the beginning to the end is known as the concentration gradient. 2. Discuss the similarities and differences between diffusion and osmosis. The Difference Between Osmosis and Diffusion Eddie Meyer Niki Patil Caleb Snider Diffusion! • Diffusion- the process by which molecules spread from areas of higher concentration, to lower concentration. Osmosis • Osmosis- is the diffusion of water across a membrane. The Difference Between Osmosis and Diffusion • Osmosis- Only water can undergo osmosis. • Diffusion- Can involve any chemical moving from one place to another. The Similarities Between Osmosis and Diffusion • Both are types of passive transport because no energy is required to move the molecules. • They are both equilibrium related processes. Works Cited Document • http://www.biologycorner.com/bio1/diffusion.html • http://sciweb.henryford.cc.mi.us/biology/jacobs/bio131 /diffusion/Diff&Os.html • Google Images • http://www.helium.com/items/1674518-thedifferences-between-diffusion-and-osmosis 3. • What would happen to a cell if placed in the following solutions (describe and illustrate). – Isotonic solution: – Hypotonic solution: – Hypertonic solution: Adam Knox, Kayla Halterman, Alexandra Young, and Emily Jones Isotonic Solution: A solution that has the same salt concentration as the normal cells of the body and the blood. When a cell is placed in an isotonic solution, the water diffuses into and out of the cell at the same rate. Hypotonic Solution: A solution that has a lower salt concentration as the normal cells of the body and the blood. When a cell is placed in a hypotonic solution, the water diffuses into the cell, causing the cell to swell and possibly explode Hypertonic Solution: A solution that has a higher salt concentration as the normal cells of the body and the blood. When a cell is placed in a hypertonic solution, the water diffuses out of the cell, causing the cell to shrivel up. 4. • Use pictures to illustrate and arrows to show the direction of osmosis for each of the conditions below. Assume the membrane is not permeable to sucrose. • Intravenous solutions must be prepared so that they are isotonic to red blood cells. A 0.9 % salt solution is isotonic to red blood cells. – Explain what will happen to a red blood cell placed in a solution of 99.3% water and 0.7% salt. – Explain what would happen to a red blood cell placed in a solution of 90% water and 10% salt. Blood Water being pulled in. Homeostasis occurs. Water comes in and out. Water being flushed out. When there is less salt inside the cell than there is around it, the cell swells, and flushes water out. When there is more salt than water, the cell will shrink and try to pull more salt in. These processes occur by the use of osmosis and diffusion. 5. • Draw and describe a plant cell in a hypotonic solution. How will a plant cell respond differently than an animal cell? Why? 5 Draw and describe a plant cell in a hypotonic solution. How will a plant cell respond differently than an Hypotonic Having deficient tone or tension Having a lower osmotic pressure than a surrounding medium or a fluid under comparison Osmotic The diffusion of fluids through membranes or porous patricians Onion Cell before and after being put in a Hypotonic Solution Before After Hypotonic Solutions abosorbs so much water out of the water out of cells that they may burst open. The plant cells usually do not explode Animal Cells Mostly do Explode Because: They don’t have a cell wall to keep the structure intact, but plant cells do 6. • For the most part, plants and animal live in either a salt water environment or a fresh water environment, not both. Explain this using the principles of diffusion. Diffusion with Fish Charles Salo Bente Bouthier Loretta Wendel Hadlee Swope http://physioweb.med.uvm.edu/diffusion/ MultiWithMembPages.htm For the most part, plants and animal live in either a salt water environment or a fresh water environment, not both. Explain this using the principles of diffusion. Our Answer Fish drink water. Salt draws water to it. In freshwater fish do not have to drink because their bodies are saltier than the water, and draw water in. Saltwater fish need to because the water is saltier than their bodies, and draws water out. What that means If freshwater and saltwater fish were to switch environments they would die. A Saltwater fish would shrivel up and die, while a freshwater fish would inflate and waterlog itself. 7. • What is required for active transport to occur? • Primary Active transport uses chemical energy such as ATP • Secondary Active Transport uses an electrochemical gradient • Active Transport uses energy as opposed to Passive Transport which uses no energy Videos: • http://sciencestage.com/v/639/cellmembrane-active-transport.html • http://www.dnatube.com/video/1589/CellMembrane-Active-Transport 8. • What if there is a large food particle or organism that a predatory cell – like an amoeba – wants to eat? It must use a process called exocytosis. Draw a diagram showing how this process works. Give two examples of substances that a cell might export this way. By: William Anderson Mikayla Cregg Jordan Kraft Exocytosis is the movement of substances out of a cell. These substances can include hormones, proteins, and waste products. • http://www.maxanim.com/physiology/Endo cytosis%20and%20Exocytosis/Endocytosi s%20and%20Exocytosis.htm Cell Transport • Active • Passive • Diffusion – Facilitated Diffusion • • • • • Osmosis Isotonic Hypertonic Hypotonic Endocytosis Osmosis: Diffusion of Water • The diffusion of water across a selectively permeable membrane is called osmosis. Real- World Applications • Plant Cells: Cellular Respiration • Preserving Fruit and Meat • Medicine – IV – Storage of Red Blood Cells When comparing two solutions to one another, we define: A. Hypotonic solution has a lower solute concentration B. Hypertonic solution has a higher solute concentrations Isotonic – solutions with equal concentration. Osmosis Simulation