HypotonicHypertonicAndIsotonic Sept 24
advertisement

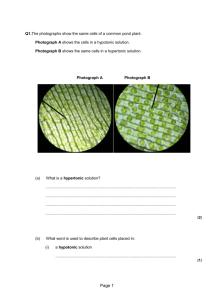
The effects of hypotonic, hypertonic and isotonic solution on animal and plant cells. • Hypertonic - Concentration with higher solute concentration and less water concentration • Hypotonic - lower solute concentration and more water concentration • Isotonic - Solution in which water molecule and solute molecule are equal in concentration. Animal and plant cell In an isotonic solution • Isotonic solution is a solution in which the concentration of solutes is equal, so: - Water diffuses into and out of the cell at equal rates. - There’s no net movement of water across the plasma membrane - The cells retain their normal shape Animal and plant cells in a hypotonic solution • Solution which contain higher concentration of water and lower concentration of solutes is called as hypotonic solution. • Since the concentration of water is higher outside the cell, there is a net movement of water from outside into the cell. • Cell gains water, swells and the internal pressure increases. Eventually burst (haemolysis). The effects of hypertonic solution in animal and plant cell • Contain higher concentration of solutes and less of water than a cell. • Since the concentration of water is higher within the cell, there is a net movement of water from inside to outside of the cell. (water leaves the cell by osmosis) • Causes the cell to shrink as its internal pressure decreases. Hypertonic solution on plant cell • Water diffuses out of the large central vacuole by osmosis. Water lose from both vacuole and cytoplasm cause to shrink. • Plasma membrane pulls away from the cell wall. (plasmolysis). • Become flaccid and less turgid. • Cell wall doesn’t shrink because it is strong and rigid. • If plasmolysis continues, death may result. • If we placed the plasmolysed plant cell in a hypotonic solution (pure water), water moves into the cell by osmosis and become turgid again. (deplasmolysis) Food preservation • The concept of osmosis and diffusion are applied in the preservation of food, such as fruits, fish and vegetables by using preservatives (salt, sugar/ vinegar) • Salt solution of hypertonic to tissue of fish. So water leaves the fish tissue and enter the salt solution by osmosis. • Fish become dehydrated and cell crenate. Therefore, bacteria can’t grow in fish tissue and bacteria cell will crenate. • Preserved fish don’t decay so soon and last longer. • Preservation with vinegar • Mangoes are soaked in vinegar which has low pH, vinegar diffuses into the tissues of the mangoes and become acidic. • Low pH prevents the growth of microorganism in mangoes and preserved mangoes can last longer.