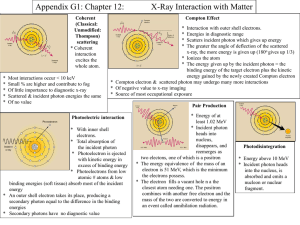
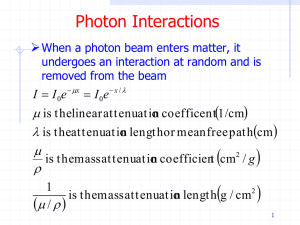
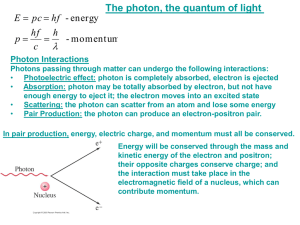
Chapter 27 Early Quantum Theory and Models of the Atom 27.1
advertisement
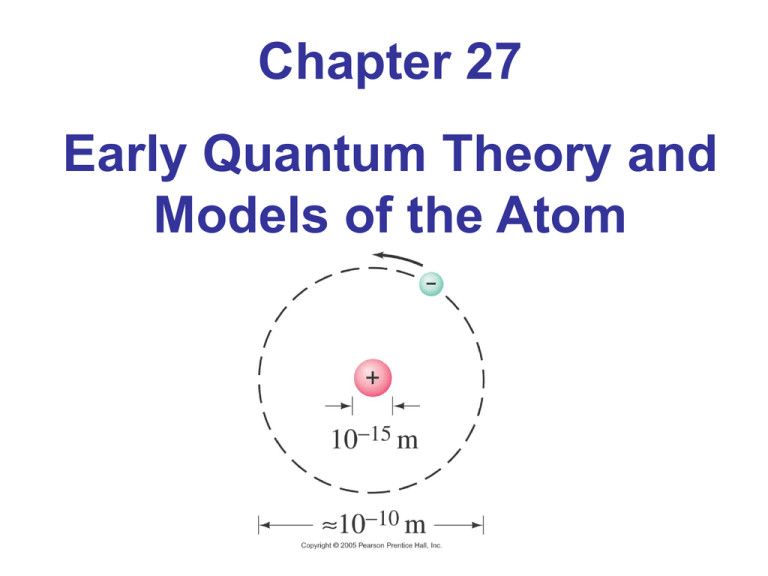
Chapter 27 Early Quantum Theory and Models of the Atom 27.1 Discovery and Properties of the Electron In the late 19th century, discharge tubes were made that emitted “cathode rays.” 27.1 Discovery and Properties of the Electron It was found that these rays could be deflected by electric or magnetic fields. Centripetal & Electric forces x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x x What happens to the charge particle as it enters the B field? v Fb qv B v2 Fc m r If an electric field is turn on, the particles direction can be straighten out. FE q E Sometimes it is convenient to express the path of a particle in terms of its charge to mass ratio e v m Br e E 2 m B r e is the charge on an electron Properties of the Electron By accelerating the rays through a known potential and then measuring the radius of their path in a known magnetic field, the charge to mass ratio could be measured: The result is Demonstration • Bring in CRT monitor with magnet. Millikan’s Oil-drop experiment The force due to gravity (mg) was balanced by electric force created by an electric field. The mass and charge of each droplet were measured Analysis showed that the charge was always an integral multiple of a smallest charge, e. Quantized energy levels • Ramp versus stair analogy. On a ramp, a box can have continuous values of potential energy. • On the stairs, the box can have only discrete (quantized) values of energy Photon Theory of Light and the Photoelectric Effect Einstein suggested that, given the success of Planck’s theory, light must be emitted in small energy packets: E hf These tiny packets, or particles, are called photons. Quantum number Planck proposed that energy of any molecular vibration can only be a whole number of hf E nhf , n 1, 2,3 n is the quantum number or number of photons h is Planck’s constant h 6.626 x1034 Js Photoelectric Effect The photoelectric effect: When light strikes a metal, electrons are emitted. Photon Theory of Light and the Photoelectric Effect Recall chapters 23 & 24 explained reflection, diffraction, and interference using ray diagrams and the theory that light behaves as a wave If light is a wave, theory predicts: Number of electrons and their energy should increase with intensity Frequency would not matter Photon Theory of Light and the Photoelectric Effect If light is particles, theory predicts: Increasing intensity increases number of electrons but not energy Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency There is a cutoff frequency below which no electrons will be emitted, regardless of intensity Photon Theory of Light and the Photoelectric Effect KE hf A photon with a frequency of red light strikes an electron, but only excites it to a higher energy state. A photon with a frequency of green light strikes an electron with enough energy to release it from the metal, but it has no KE after being released A photon with a frequency of blue light strikes an electron and releases with some velocity. Photon Theory of Light and the Photoelectric Effect KE hf hf : is the energy of the incoming photon ɸ :is the work function, the energy required to break the electron free KE : is the kinetic energy of the released electron Increasing the intensity of light (100W bulb vs. 60W bulb) KE hf Increasing the intensity of light does not increase the energy of the photon. It does increase the number of photons, which increases the number of ejected electrons (more current). Photon Theory of Light and the Photoelectric Effect The particle theory assumes that an electron absorbs a single photon. Plotting the kinetic energy vs. frequency: y = 0.4151x - 2.3575 3 KE (eV) Max 2.5 2 1.5 1 0.5 0 0 2 4 6 8 Frequency (E14 Hz) 10 12 14 If the photon doesn’t have sufficient energy to release the electron, it can be “excited” to a higher energy state. The electrons in free atoms can only be found in only certain discrete energy states. When the electrons fall back to the “ground state”, they release a very specific spectrum of light Hydrogen Spectrum 27.12 The Bohr Atom The lowest energy level is called the ground state; the others are excited states. 2 Z E 2 n Z is the # of Charges n is the Energy State Energy, Mass, and Momentum of a Photon When objects travel close to or at the speed of light, relativistic equations for length, time and momentum must be observed 2 v L Lo 1 2 c T To 1 P 2 v c2 mo v v2 1 2 c Photons travel at the speed of light. If v = c, then the denominator is zero which can only happen if the rest mass of the photon is zero. This means a photon can never be at rest Compton Effect This is another effect that is correctly predicted by the photon model and not by the wave model. Compton Effect Compton found that the scattered X-rays had a slightly longer wavelength than the incident ones, and that the wavelength depended on the scattering angle: This means the exiting photon has less energy Photon Interactions; Pair Production In pair production, energy, electric charge, and momentum must all be conserved. The photon disappears and creates an electron-positron pair. Rest mass is being created from pure energy: E=mc2 In pair production, energy, electric charge, and momentum must all be conserved. Energy is conserved through the mass and kinetic energy of the electron and positron Charge is conserve by creating both a positive and negative charge. The interaction must take place in the electromagnetic field of a nucleus, which conserves momentum. Photon Interactions; Pair Production Photons passing through matter can undergo the following interactions: 1. Photoelectric effect: photon is completely absorbed, electron is ejected 2. Photon may be totally absorbed by electron, but not have enough energy to eject it; the electron moves into an excited state 3. The photon can scatter from an atom and lose some energy 4. The photon can produce an electron-positron pair. Wave-Particle Duality; The Principle of Complementarity Phenomena such as diffraction and interference show that light is a wave Phenomena such as the photoelectric effect and the Compton effect that show that it is a particle. Which is it? This question has no answer; we must accept the dual wave-particle nature of light. Wave Nature of Matter Just as light sometimes behaves as a particle, matter sometimes behaves like a wave. The wavelength of a particle of matter is: Early Models of the Atom Rutherford’s model of the atom is mostly empty space: 27.11 Atomic Spectra: Key to the Structure of the Atom An atomic spectrum is a line spectrum – only certain frequencies appear. If white light passes through such a gas, it absorbs at those same frequencies. Atomic Spectra: Key to the Structure of the Atom A portion of the complete spectrum of hydrogen is shown here. The lines cannot be explained by the Rutherford theory. The Bohr Atom Bohr proposed that the possible energy states for atomic electrons were quantized – only certain values were possible. Then the spectrum could be explained as transitions from one level to another. Summary of Chapter 27 • Planck’s hypothesis: molecular oscillation energies are quantized • Light can be considered to consist of photons, each of energy • Photoelectric effect: incident photons knock electrons out of material Summary of Chapter 27 • Compton effect and pair production also support photon theory • Wave-particle duality – both light and matter have both wave and particle properties • Wavelength of an object: Photon absorption and Emission