Photoelectric Effect
advertisement

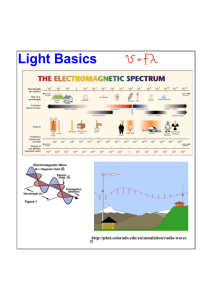
Honors Chemistry Unit 3 - Electrons Lesson 2 – Photoelectric Effect & Wave Formulas Book Section: 4-1 Do Now: Prepare for Quiz 3-1. Objective: SWBAT use wave formulas to solve problems w/ frequency & wavelength. Agenda: Quiz, Lesson, Videos, Element of the Day Electromagnetic Radiation All electromagnetic radiation travels at the same velocity: the speed of light (c). c = 3.00 x 108 m/s c Practice The yellow light given off by a sodium vapor lamp used for public lighting has a wavelength of 589 nm. What is the frequency of this radiation? Practice The yellow light given off by a sodium vapor lamp used for public lighting has a wavelength of 589 nm. What is the frequency of this radiation? 5.09 x 1014 s-1 (or Hz) Photoelectric Effect Problem with light as a wave – does not explain the photoelectric effect. Albert Einstein – won Nobel Prize for showing that the color of light determines energy, not intensity. VIDEO: Photoelectric Effect Photoelectric Effect The only way to rationalize that the color matters more than intensity was to think of light as a “packet,” or particle of light called a photon. Blue photons had more energy than red photons. You can shoot a machine gun against a brick wall, but the wall won’t fall. You need a wrecking ball. Lots of red photons won’t generate a current, but a few blue ones will. Light behaves as both a wave and as a particle…… strange… HW 3-2: #24-25 This Week: Thursday: The Mole & Energy of Light (4-1), Quiz 3-2 Friday: The Bohr Model of the Atom (4-1), Quiz 3-3 Likely Unit 3 Test Date: Tuesday, October 19