Photons, Electrons, and Atoms
advertisement
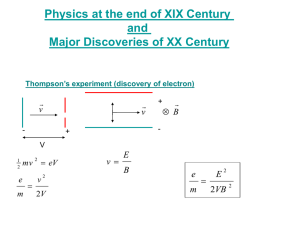
Photons, Electrons, and Atoms Visible and non-visable light • Frequencies around 1015 Hz • Much higher than electric circuits • Theory was about vibrating charge in the atom • This lead to the “quantum” theory of light • It states that some of the properties of EM radiation resemble those of particles • Where KE is proportional to f The Photoelectric Effect • It is caused by an element absorbing certain wavelength of light which causes electrons to be ejected from the surface • This “potential energy barrier” can be found with the work function f • Classic mechanics predicted that the amplitude of the wave, not its frequency, would cause more electrons to be emitted Photoelectric cont. • It was found that the amplitude, no matter how bright, would not cause the electrons to leave. • Only when critical or threshold frequency was reached, would the electrons move • For most metals it’s in the ultraviolet range • At frequencies higher than ft, the stopping potential would completely stop the flow of electrons from the surface Equations • Stopping potential 1 2 m vmax eV0 2 • Energy of a photon E hf • Planck’s constant h = 6.6260755 x10-34 j.s 1 2 m vmax hf f eV0 2 The eV • The electron volts relates electrons and energy • 1 eV = 1.602 x 10-19 J • Ex: If Vo = 5V then eV = 5eV • Example 41-1 hc • Photon energy E hf • p = momentum E pc p h EM Spectrum Line spectra • Line spectra phenomenon vs spectrum • Each line is an image of the spectrograph slit, deviated at an angle that depends on the frequency of the light • Each element emits a “signature” • Certain wavelengths of light unique to its atomic structure • Classical mechanics could not explain this Energy • Bohr proposed the idea of energy levels • All atoms of an element have the same energy levels, with no electrons existing in “intermediate” levels • The electrons can “jump” from a higher to a lower level by emitting a photon • hf = Ei - Ef Balmer Series • Trial and error resulted in the formula • R = 1.097 x 107 m-1 • Substituting in for we get En ( energy levels 1 1 R 2 2 2 n 1 hcR En 2 n Emission spectra Sources • http://cwx.prenhall.com/bookbind/pubbook s/hillchem3/medialib/media_portfolio/07.ht ml