lecture25
advertisement

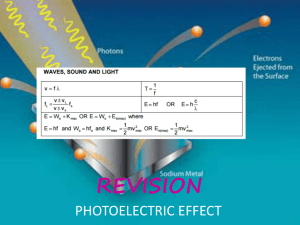
Physics at the end of XIX Century and Major Discoveries of XX Century Thompson’s experiment (discovery of electron) v - v + B - + V 1 2 mv e m 2 eV v 2 2V v E B e m E 2 2VB 2 Emission and absorption of light Spectra: •Continues spectra •Line spectra Three problems: •“Ultraviolet catastrophe” •Photoelectric effect •Michelson experiment Continues spectra and “Ultraviolet catastrophe” Stefan-Boltzmann law for blackbody radiation: I I T 4 Wien displacement law: I max T 2 . 90 10 3 mK Rayleigh’s law: I 2 ckT 4 Plank’s law: I 2 hc e 5 hc kT 1 Plank’s constant: h 6 . 62 10 34 J s 4 . 14 10 15 eV s E hf Example 1: What is the wavelength the frequency corresponding to the most intense light emitted by a giant star of surface temperature 5000 K? max T 2 . 90 10 max 2 . 90 10 3 3 mK m K / 5000 K 0 . 580 10 f max c / max 3 10 m / s / 0 . 580 10 8 6 6 m 580 nm m 5 . 2 10 14 Hz Example 2: What is the wavelength the frequency of the most intense radiation from an object with temperature 100°C? max 2 . 90 10 3 m K / 273 100 K 7 . 77 10 f max c / max 3 10 m / s / 7 . 77 10 8 6 6 m 3 . 9 10 m 7 . 77 m 13 Hz Photoelectric effect light Experiment: A If light strikes a metal, electrons are emitted. The effect does not occur if the frequency of the light is too low; the kinetic energy of the electrons increases with frequency. Classical theory can not explain these results. If light is a wave, classical theory predicts: • Frequency would not matter • Number of electrons and their energy should increase with intensity Quantum theory: Einstein suggested that, given the success of Planck’s theory, light must be emitted and absorbed in small energy packets, “photons” with energy: E hf If light is particles, theory predicts: • Increasing intensity increases number of electrons but not energy • Above a minimum energy required to break atomic bond, kinetic energy will increase linearly with frequency • There is a cutoff frequency below which no electrons will be emitted, regardless of intensity light Photoelectric effect (quantum theory) Photons! Plank’s constant: E hf A 1 2 h 6 . 62 10 34 J s mv max hf W 0 2 (1) K max E W 0 I eV 0 1 2 mv max K max 2 eV 0 hf-W 0 V -V0 V0 V0 V0 f fmin f h f- e W0 e h e hf min W 0 Photons: E pc hf - energy p hf c h - momentum (2) Example: The work function for a certain sample is 2.3 eV. What is the stopping potential for electrons ejected from the sample by 7.0*1014 Hz electromagnetic radiation? W 0 2 . 3 eV f 7 . 0 10 14 eV 0 hf-W Hz V0 ? V0 0 V0 4 . 14 10 15 hf W 0 e eV s 7 . 0 10 14 Hz 2 . 3 eV 0 . 6V 1e Example: The work function for sodium, cesium, copper, and iron are 2.3, 2.1, 4.7, and 4.5 eV respectively. Which of these metals will not emit electrons when visible light shines on it? f 7 . 5 10 W0 ? 14 Hz hf min W 0 W 0 4 . 14 10 15 eV s 7 . 5 10 14 Copper, and iron will not emit electrons Hz 3 . 1eV