E 1
advertisement

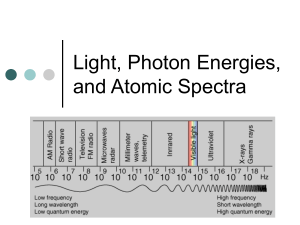
Early Quantum Mechanics Chapter 27 Three Major Discoveries Light is both a Electron wave and a Orbitals are particle Quantized Planck Einstein Compton Bohr The electron (and all matter) is both a wave and a particle De Broglie (Later Schrodinger/Hei senberg) Wein’s Law • Treat’s light solely as a wave • Hot “blackbodies” radiate EM • The hotter the object, the shorter the peak wavelength • Sun (~6000 K) emits in blue and UV • A 3000 K object emits in IR 2.90 X 10-3 mK = lpeakT Wein’s Law: Ex 1 Estimate the temperature of the sun. The Suns light peak at about 500 nm (blue) 500 nm = 500 X 10-9 m T = 2.90 X 10-3 mK lpeak T = 2.90 X 10-3 mK = 6000 K 500 X 10-9 m Wein’s Law: Ex 2 Suppose a star has a surface temperature of about 3500 K. Estimate the wavelength of light produced. 2.90 X 10-3 mK = lpeakT l peak = 2.90 X 10-3 mK T l peak = 2.90 X 10-3 mK = 8.29 X 10-7 m = 830 nm 3500 K Planck’s Quantum Hypothesis • Energy of any atomic or molecular vibration is a whole number • Photon – the light particle • Photons emitted come in “packets” • E = hf • h = 6.626 X 10-34 J s (Planck’s constant) Photons: Ex 1 Calculate the energy of a photon of wavelength 600 nm. 600 nm X 1 X 10-9m = 6 X10-7 m 1 nm c = lf f = c/l = (3X108 m/s)/(6 X10-7 m) = 5 X 1014 s-1 E = hf E = (6.626 X 10-34 J s)(5 X 1014 s-1) = 3.3 X 10-19 J Photons: Ex 1a Convert your answer from the previous problem to electron Volts (1 eV = 1.6 X 10-19 J) Photons: Ex 2 Calculate the energy of a photon of wavelength 450 nm (blue light). Photons: Ex 2a Convert your answer from the previous problem to electron Volts (1 eV = 1.6 X 10-19 J) Photons: Ex 3 Estimate the number of photons emitted by a 100 Watt lightbulb per second. Assume each photon has a wavelength of 500 nm. 500 nm X 1 X 10-9m = 5 X10-7 m 1 nm c = lf f = c/l = (3X108 m/s)/(5 X10-7 m) = 6 X 1014 s-1 100 Watts = 100 J/s (we are looking at 1 second) E = nhf n = E/hf n = 100 J (6.626 X 10-34 J s)(6 X 1014 s-1) n = 2.5 X 1020 Photoelectric Effect (Einstein) • When light shines on a metal, electrons are emitted • Can detect a current from the electrons • Used in light meter, scanners, digital cameras (photodiodes rather than tubes) Three Key Points 1. Below a certain frequency, no electrons are emitted 2. Greater intensity light produces more electrons 3. Greater Frequency light produces no more electrons, but the come off with greater speed Low Frequency Not enough energy to eject electron High Frequency Can eject electron Energy of photon is greater than W (ionization energy 2. More intensity – More photons – More electrons ejected with same KE 3. Greater Frequency – No more electrons ejected – Electrons come off with greater speed (KE) hf = KE + W hf = energy of the photon KE = Maximum KE of the emitted electron W = Work function to eject electron Metal Na Al Cu Zn Ag Pt Pb Fe Work Function (eV) 2.46 4.08 4.70 4.31 4.73 6.35 4.14 4.50 hf = KE + W: Ex 1 What is the maximum kinetic energy of an electron emitted from a sodium atom whose work function (Wo) is 2.28 eV when illuminated with 410 nm light? 410 nm = 410 X 10-9 m or 4.10 X 10-7 m 2.28 eV X 1.60 X 10-19J 1 eV = 3.65 X 10-19 J c = lf f = c/l f = c/(4.10 X 10-7 m) = 7.32 X 1014 s-1 hf = KE + W KE = hf – W KE = (6.626 X 10-34 J s)(7.32 X 1014 s-1) - 3.65 X 10-19 J KE = 1.20 X 10-19 J or 0.75 eV hf = KE + W: Ex 2 What is the maximum kinetic energy of an electron emitted from a sodium atom whose work function (Wo) is 2.28 eV when illuminated with 550 nm light? ANS: 2.25 eV hf = KE + W: Ex 3 What is the maximum wavelength of light (cutoff wavelength) that will eject an electron from an Aluminum sample? Aluminum’s work function (Wo) is 4.08 eV? 4.08 eV X 1.60 X 10-19J 1 eV = 6.53 X 10-19 J hf = KE + W hf = 0 + W (looking for bare minimum) c = lf f = c/l hf = W hc = W l l = hc = (6.626 X 10-34 J s)(3.0 X 108 m/s) W 6.53 X 10-19 J l = 3.04 X 10-7 m = 304 nm (UV) Photon/Matter Interactions 1. Electron excitation (photon disappears) 2. Ionization/photoelectric effect (photon disappears) 3. Scattering by nucleus or electron 4. Pair production (photon disappears) Electron Excitation Ionization/Photoelectric Effect •Photon is absorbed (disappears) •Photon is absorbed (disappears) •Electron jumps to an excited state •Electron is propelled out of the atom Scattering •Photon collides with a nucleus or electron •Photon loses some energy •Speed does not change, but the wavelength increases Pair Production •Photon closely approaches a nucleus •Photon disappears •An electron and positron are created. 3. Scattering: Compton Effect • Electrons and nuclie can scatter photons • Scattered photon is at a lower frequency than incident photon • Some of the energy is transferred to the electron or nucleus l’ = l + h (1 – cos q) moc l’ = wavelength of scattered photon l = wavelength of incident photon mo = rest mass of particle q = angle of incidence Compton Effect: Ex 1 X-rays of wavelength 0.140 nm are scatterd from a block of carbon. What will be the wavelength of the X-rays scattered at 0o? l’ = l + h (1 – cos q) moc l’ = 140 X10-9m + (6.626 X 10-34 J s)(1 – cos 0) (9.11 X 10-31 kg)(3 X 108m/s) l’ = 140 X10-9m + 0 l’ = 140 nm Compton Effect: Ex 2 What will be the wavelength of the X-rays scattered at 90o? l’ = l + h (1 – cos q) moc l’ = 140 X10-9m + (6.626 X 10-34 J s)(1 – cos 90) (9.11 X 10-31 kg)(3 X 108m/s) l’ = 140 X10-9m + 2.4 X 10-12 m l’ = 142 nm Compton Effect: Ex 3 What will be the wavelength of the X-rays scattered at 180o? l’ = l + h (1 – cos q) moc l’ = 140 X10-9m + (6.626 X 10-34 J s)(1 – cos 180) (9.11 X 10-31 kg)(3 X 108m/s) l’ = 140 X10-9m + 4.8 X 10-12 m l’ = 145 nm (straight back) 4. Pair Production • Photon Disappears • e- and e+ are produced • They have opposite direction (law of conservation of momentum) • When e- and e+ collide they annihilate each other a new photon appears Principle of Complimentarity • Neils Bohr • Any experiment can only observe light’s wave or particle properties, not both • Different “faces” that light shows Wave Particle Prism Blackbody Radiation Photoelectric effect The Discovery of the Electron (Thomson) • Cathode Ray Tube • Charged particles produced (affected by magnetic field) • Concluded that atom must have positive and negative parts • Electron – negative part of the atom • Only knew the e/m ratio • Plum Pudding Model Charge and Mass of the Electron (Millikan) • Oil drop experiment • Determines charge on electron (uses electric field to counteract gravity) • Quantized • e = 1.602 X 10-19 C • m = 9.11 X 10-31 kg The Nucleus (Rutherford) • Gold Foil Experiment • Discovers nucleus (disproves Plum Pudding Model) • Planetary Model Wave Nature of Matter • Everything has both wave and particle properties • DeBroglie Wavelength E2 = p2c2 + m2c4 E2 = p2c2 E = pc E = hf hf = pc c = lf (consider a photon) (photon has no mass) hf = plf p = mv (for a particle) hf = mvlf h = mvl l= h mv Everything has a wavelength Diffraction pattern of electrons scattered off aluminum foil DeBroglie Wavelength: Ex 1 Calculate the wavelength of a baseball of mass 0.20 kg moving at 15 m/s l= h mv l = (6.626 X 10-34 J s) (0.20 kg)(15 m/s) l = 2.2 X 10-34 m DeBroglie Wavelength: Ex 2 Calculate the wavelength of an electron moving at 2.2 X 106 m/s l= h mv l = (6.626 X 10-34 J s) (9.11 X 10-31 kg)(2.2 X 106 m/s) l = 3.3 X 10-10 m or 0.33 nm DeBroglie Wavelength: Ex 3 Calculate the wavelength of an electron that has been accelerated through a potential difference of 100 V V = PE q V = 1 mv2 2 q v2 = 2qV/m (PE =KE) v = (2qV/m)1/2 v=[(2)(1.602 X 10-19 C)(100V)/(9.11 X 10-31 kg)]1/2 v = 5.9 X 106 m/s l= h mv l = (6.626 X 10-34 J s) (9.11 X 10-31 kg)(5.9 X 106 m/s) l = 3.3 X 10-10 m or 0.33 nm Electron Microscope • Electron’s wavelength is smaller than light • Magnetic focusing DNA Line Spectra • Discharge tube – Low density gas (acts like isolated atoms) – Run a high voltage through it – Light is emitted • Light is emitted only at certain (discrete) wavelengths • Gases absorb light at the same frequency that they emit Hydrogen Helium Solar absorption spectrum Explaining the Lines Lyman Series 1 = R1 l 12 1 n2 Balmer Series 1 = R1 l 22 1 n2 Paschen Series 1 = R1 1 l 32 n2 Bohr Model: Hydrogen • Electrons orbit in ground state (without radiating energy) • Classically, electrons should radiate energy since that are accelerating because they are changing directions • Jumps to excited state by absorbing a photon • Returns to ground state by emitted a photon Bohr Model hf = Ee - Eg Bohr’s Equation • Worls only for H and other 1electron atoms (He+, Li2+, Be3+, etc…) • Energy of ionized atom is set at 0 • Orbital energies are below zero En = -13.6 eV n2 En = Energy of an orbital -13.6 eV = Orbital of hydrogen closest to nucleus n = Number of the orbital (1 eV = 1.60 X 10-19 J) Bohr’s Equation: Ex 1 Calculate the energy of the first three orbitals of hydrogen En = -13.6 eV n2 E1 = -13.6 eV 12 E1 = -13.6 eV E2 = -13.6 eV 22 E2 = -3.4 eV E3 = -13.6 eV 32 E3 = -1.51 eV Bohr’s Equation: Ex 2 What wavelength of light is emitted if a hydrogen electron drops from the n=2 to the n=1 orbit? E1 = -13.6 eV E2 = -3.4 eV DE = (-3.4eV - -13.6 eV) = 10.2 eV DE = (10.2 eV)(1.60 X 10-19J/eV) = 1.63 X 10-18J DE = hf f = c/l DE = hc/l l = hc DE l = (6.626 X 10-34 J s)(3.0 X 108 m/s) (1.63 X 10-18J) l = 1.22 X 10-7 m = 122 nm (UV) Bohr’s Equation: Ex 3 Calculate the wavelength of light emitted if a hydrogen electron drops from the n=6 to the n=2 orbit? (ANS: 410 nm (violet)) Bohr’s Equation: Ex 4 Calculate the wavelength of light that must be absorbed to exite a hydrogen electron from the n=1 to the n=3 orbit? (ANS: 103 nm (UV)) Bohr Model: Other Atoms En = (Z2)(-13.6 eV) n2 Z = Atomic number of the element (H=1, He=2, etc) Other Atoms: Ex 1 Calculate the ionization energy of He+. This is the energy needed to move an electron from n=1 to zero. E1 = (22)(-13.6 eV) 12 E1 = -54.4 eV Other Atoms: Ex 2 What wavelength of light would be required to ionize He+ E1 = -54.4 eV 8.70 X 10-18 J E = hf E = hc/l l = hc = (6.626 X 10-34 J s)(3.0 X 108 m/s) E (8.70 X 10-18 J) l = 22.8 nm Other Atoms: Ex 3 Calculate E1 for a Li2+ ion. E1 = (32)(-13.6 eV) 12 E1 = -122.4 eV Other Atoms: Ex 4 What wavelength of light would be emitted from a n=3 to n=1 transition in He+ ? (ANS: l = 34.2 nm) Wave/Particle Duality DeBroglie • Each e- is actually a standing wave • Only certain wavelengths produce resonance Forbidden Zone Circumference = 2pr 2prn = nl l= h mv mvrn = nh 2p