Chapter 28
advertisement

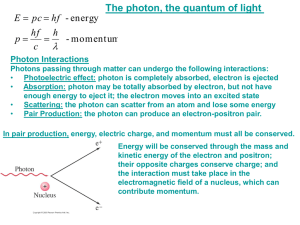
AP Physics Chapter 28 Quantum Mechanics and Atomic Physics Chapter 28: Quantum Mechanics and Atomic Physics 28.1 28.2-5 Quantization: Planck’s Hypothesis Omitted Homework for Chapter 28 • Read Chapter 28 • HW 28: p. 889: 3-6, 9, 12, 15. 28.1: Matter Waves: The De Broglie Hypothesis Photon Momentum • A photon is a massless particle which carries energy. • Photon energy can be written E = hf = hc/ • The momentum of a photon carries is related to its wavelength by p = E = hf = h c c On Gold Sheet • The energy in electromagnetic waves of wavelength can be thought of as being carried by photon particles, each having a momentum of h/. • Louis de Broglie (1892 – 1987) – French physicist and Nobel laureate • De Broglie speculated that if light sometimes behaves like a particle, perhaps material particles, such as electrons, also have wave properties. • In 1924 de Broglie hypothesized that a moving particle has a wave associated with it. The wavelength of the particle is related to the particle’s momentum (p=mv). = h =_h_ p mv On Gold Sheet • These waves associated with moving particles were called matter waves or, more commonly de Broglie waves. Question: If particles really are associated with a wave, why then have we never observed wave effects such as diffraction or interference for them? Example 28.1: What is the wavelength of the matter wave associated with a) a ball of mass 0.50 kg moving with a speed of 25 m/s? b) an electron moving with a speed of 2.5 x 107 m/s? The wavelength of the ball is much shorter than that of the electron. That is why matter waves are important for small particles like electrons since their wavelengths are comparable to the sizes of the objects they interact with. It is easier to observe interference and diffraction for electrons than the ball and all other everyday objects. Problem Solving Hint: Accelerating an Electron Through a Potential Difference, V • Since p = mv and KE = ½ mv2, KE = p2 2m • By energy conservation, KE = UE = qV = eV • So, p2 = eV 2m or p = 2meV • For these conditions, the de Broglie wavelength is = h = h = p 2meV • Substitute in the numbers for h, e, and m: = = 1.50 V nm 1.50 V x 10-9 m where V is in volts. h2___ 2meV Example 28.2: An electron is accelerated by a potential difference of 120 V. a) What is the wavelength of the matter wave associated with the electron? b) What is the momentum of the electron? c) What is the kinetic energy of the electron? • In 1927, two physicists in the US, C.J. Davisson and L.H. Germer, used a crystal to diffract a beam of electrons, thereby demonstrating a wavelike property of particles. • In order to test de Broglie’s hypothesis that matter behaved like waves, Davisson and Germer set up an experiment very similar to what might be used to look at the interference pattern from x-rays scattering from a crystal surface. The basic idea is that the planar nature of crystal structure provides scattering surfaces at regular intervals, thus waves that scatter from one surface can constructively or destructively interfere from waves that scatter from the next crystal plane deeper into the crystal. Video: http://www.tutorvista.com/content/physics/physics-iv/radiation-andmatter/davisson-and-germer-experiment.php Simulation: http://phet.colorado.edu/en/simulation/davisson-germer •This simple apparatus send an electron beam with an adjustable energy to a crystal surface, and then measures the current of electrons detected at a particular scattering angle theta. The results of an energy scan at a particular angle and an angle scan at a fixed energy are shown below. Both show a characteristic shape indicative of an interference pattern and consistent with the planar separation in the crystal. This was dramatic proof of the wave nature of matter. Davisson-Germer Experiment • A single crystal of nickel was cut to expose a spacing of d = 0.215 nm between the lattice planes. • When a beam of electrons of kinetic energy 54.0 eV was directed onto the crystal face, the maximum in the intesity of the scattered electrons was observed at an angle of 50°. • According to wave theory, constructive interference due to waves reflected from two lattice planes a distance of d apart should occur at certain angles of scattering . • The theory predicts the first order maximum should be observed at an angle given by d sin = (0.215 nm) sin 50° = 0.165 nm • The de Broglie wavelength of the electrons is: = 1.50 nm • The wavelengths agree!! 1.50 nm = 0.167 nm • Another experiment carried out in the same year by G.P.Thomson in Great Britain added further proof. • Thomson passed a beam of energetic electrons through a thin metal foil. • The diffraction pattern of the electrons was the same as that of X-rays. • Particles exhibit wavelike properties…..confirmed. electron diffraction pattern X-ray diffraction pattern Check for Understanding 1. The momentum of a photon is a) zero b) equal to c c) proportional to its frequency d) proportional to its wavelength e) given by the de Broglie hypothesis Answer: c hf = pc 2. The Davisson-Germer experiment a) dealt with X-ray spectra b) confirmed blackbody radiation c) supplied further evidence for Wien’s displacement law d) demonstrated the wavelike properties of electrons Answer: d Check for Understanding 3. Homework for Chapter 28 • HW 28: p. 889: 3-6, 9, 12, 15. Formulas for Chapter 28